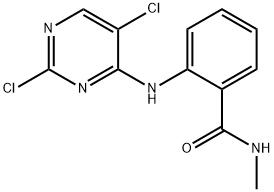
C12H10Cl2N4O synthesis
- Product Name:C12H10Cl2N4O
- CAS Number:761440-08-8
- Molecular formula:C12H10Cl2N4O
- Molecular Weight:297.14
Yield:761440-08-8 95%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in isopropyl alcohol; for 18 h;Heating / reflux;
Steps:
2
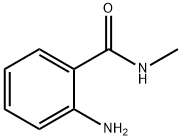
Intermediate 2; 2-[(2,5-Dichloro-4-pyrimidinyl)amino]-N-methylbenzamide A round-bottomed flask was charged with 2,4,5-trichloropyrimidine (2 g, 11.1 mmol), 2-amino-N-methylbenzamide (2 g, 13.3 mmol), di-isopropyl-ethylamine (2.3 mL, 13.3 mmol) and 40 mL isopropanol. The flask was fitted with a reflux condenser and the reaction was heated to reflux and stirred for 18 h. A white solid appeared in the reaction mixture. The reaction was cooled to room temperature, and the solid was filtered off and washed with isopropanol. After drying, the white solid (3.16 g, 10.5 mmol, 95% yield) was identified as 2-[(2,5-dichloro-4-pyrimidinyl)amino]-N-methylbenzamide. MS: M(C12H10Cl2N4O)=297.14, (M+H)+=297 and 299.
References:
US2008/182852,2008,A1 Location in patent:Page/Page column 7-8
118-48-9
406 suppliers
$5.00/5G
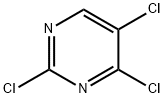
5750-76-5
352 suppliers
$5.00/1g

74-89-5
7 suppliers
$13.44/25ML

761440-08-8
21 suppliers
inquiry

118-48-9
406 suppliers
$5.00/5G

761440-08-8
21 suppliers
inquiry
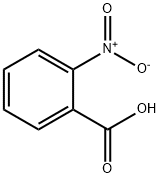
552-16-9
337 suppliers
$10.00/10g

761440-08-8
21 suppliers
inquiry
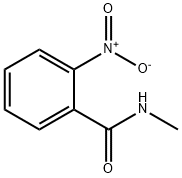
3400-29-1
6 suppliers
inquiry

761440-08-8
21 suppliers
inquiry