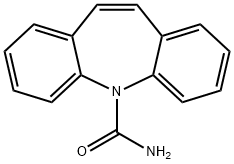
Carbamazepine synthesis
- Product Name:Carbamazepine
- CAS Number:298-46-4
- Molecular formula:C15H12N2O
- Molecular Weight:236.27
256-96-2
301 suppliers
$5.00/100mg
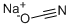
917-61-3
311 suppliers
$24.78/100g

298-46-4
708 suppliers
$7.00/1g
Yield:298-46-4 98.8%
Reaction Conditions:
in water;acetic acid at 15 - 60; for 4 h;Product distribution / selectivity;
Steps:
6
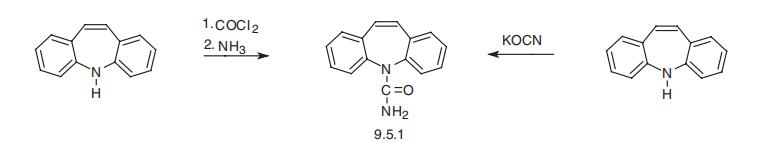
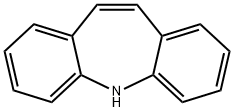
3 kg iminostilbene are stirred in a mixture of 28.5 l acetic acid and 1.5 l water, and heated to 60° C. Within about 2 hours 1.66 kg 98% sodium cyanate is added, the the mixture is cooled to 15° C. and held for a further 2 hours between 15° C. to 20° C., then the crystals are sucked off, washed with 2 l acetic acid and dried, yielding 3.39 kg (92.5% of theoretical) of the end product, having a melting point of 190° C. to 192° C.Next 22 l acetic acid was distilled off, 10 l water was added to the residue, briefly stirred, sucked off and washed with 5 l water and dried and a further 0.28 kg of the product was obtained which was recrystallized from toluene to 0.23 kg (6.3% of theoretical) having a melting point of 191° C. to 194° C. This resulted in a total yield of 98.8% of theoretical).
References:
US7015322,2006,B1 Location in patent:Page/Page column 4
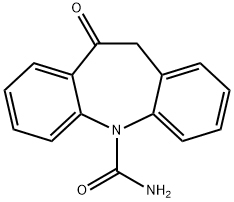
28721-07-5
586 suppliers
$5.00/50mg

298-46-4
708 suppliers
$7.00/1g

256-96-2
301 suppliers
$5.00/100mg

298-46-4
708 suppliers
$7.00/1g
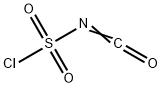
1189-71-5
364 suppliers
$18.00/5g

256-96-2
301 suppliers
$5.00/100mg

298-46-4
708 suppliers
$7.00/1g
![5-Cyano-5H-dibenz[b,f]azepine](/CAS/GIF/42787-75-7.gif)
42787-75-7
74 suppliers
$230.00/50g

298-46-4
708 suppliers
$7.00/1g