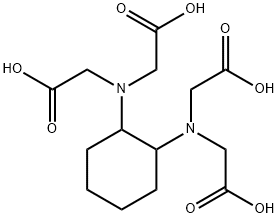
CDTA synthesis
- Product Name:CDTA
- CAS Number:482-54-2
- Molecular formula:C14H22N2O8
- Molecular Weight:346.33
Yield:-
Reaction Conditions:
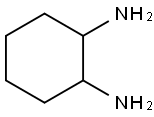
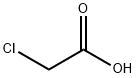
Stage #1:rac-diaminocyclohexane;chloroacetic acid with tetramethyl ammoniumhydroxide in water at 100; for 1 h;
Stage #2: with hydrogenchloride in water; pH=2
Steps:
1
EXAMPLE 1; Into a vessel equipped with parts to accommodate a thermometer, a condensing column with a reflex cap, and a graduated liquid dropping column with a stopcock were charged 85 g of chloroacetic acid and dissolved in 200 g of deionized (DI) water. The solution was cooled to 0° C. and 135 g of 10% aqueous solution of Tetramethylammonium hydroxide were added to neutralize the acid. During the neutralization the temperature did not exceed 10° C. 26 g of 1,2-diaminocyclohexane were added and the reaction mixture was stirred for 1 hour at 100° C. and then cooled to room temperature. The product was acidified slowly with 100 g of concentrated hydrochloric acid to pH 3 with cloudy solution formation. The cloudy solution was stirred for about 5 to 10 minutes. Additional concentrated hydrochloric acid was slowly added dropwise until a pH 2 was reached and a precipitate was formed. The product was filtered and 1,2-diaminocyclohexane tetraacetic acid crystals were recovered. A portion (39.9 g) of the CDTA crystals was dissolved in another vessel in 100 ml, of DI water to which was added while stirring constantly 30% aqueous ammonium hydroxide (freshly made) until all the CDTA crystals dissolved to form a clear solution at a pH 6 to 10. A diluted 18.5% hydrochloric acid was added slowly dropwise until a pH 4 was reached where the CDTA begins to recrystallize into a cloudy solution. The mixture was stirred for about 5 to 10 minutes. Again diluted to 5% hydrochloric acid was added slowly dropwise until a pH 2 was reached an complete crystallization occurs. The mixture was filtered and the CDTA recovered. The use of an ion exchange process for purifying hydroxylamine is well know. The following example shows the use of the CDTA of the present invention to stabilize an ultra-high purity grade hydroxylamine purified by an ion exchange method.
References:
Ward, Irl E.;French, Danielle US2005/131250, 2005, A1 Location in patent:Page/Page column 3