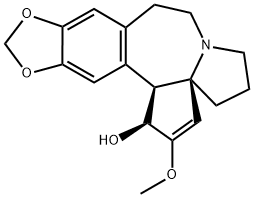
CEPHALOTAXINE synthesis
- Product Name:CEPHALOTAXINE
- CAS Number:24316-19-6
- Molecular formula:C18H21NO4
- Molecular Weight:315.36
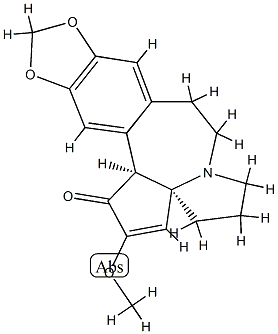
38750-57-1

24316-19-6
Sodium borohydride (27 mg, 0.70 mmol, 40 eq.) was added to a stirred solution of (-)-cephathione (S9) (5.5 mg, 18 μmol, 1.0 eq.) in methanol (0.65 mL) at -78 °C. The resulting white mixture was continued to be stirred at -78 °C for 10 minutes, followed by a slow warming to 25 °C and stirring at this temperature for 1 hour. Upon completion of the reaction, the mixture was diluted with water (30 mL) and extracted with dichloromethane (4 x 15 mL). The organic phases were combined, dried with sodium sulfate, gravity filtered, and the filtrate was concentrated by rotary evaporation to afford the target product (-)-1 (5.2 mg, 95% yield) as a light yellow film. Thin layer chromatography (TLC) showed an Rf value of 0.08 (unfolding agent: 10% methanol/dichloromethane). The structure of the product was confirmed by 1H NMR (500 MHz, CDCl3), IR and HRMS (ESI): 1H NMR δ 6.68 (s, 1H, ArH), 6.65 (s, 1H, ArH), 5.90 (s, 2H, OCH2O), 4.93 (s, 1H, vinyl H), 4.76 (d, 1H, J = 9Hz, CH(OH )), 3.73 (s, 3H, OCH3), 3.68 (d, 1H, J = 9 Hz, ArCHCH(OH)), 3.35 (m, 1H, CH2), 3.08 (m, 1H, CH2), 2.92 (dd, 1H, J = 12, 11, 7 Hz, CH2), 2.54-2.62 (m, 2H, CH2), 2.36 (ddd, 1H, J = 14, 7Hz, CH2), 2.00 (m, 1H, CH2), 1.60-1.90 (m, 4H, CH2); IR (pure film) 3411 (broad peaks, weak), 2926 (medium), 1651 (medium), 1503 (medium), 1486 (strong), 1222 (strong) cm-1; HRMS (ESI) m/z: C18H22NO4 ([M+H]+) calculated value 316.1549, measured value 316.1559.
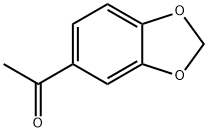
3162-29-6
215 suppliers
$18.00/5g

24316-19-6
204 suppliers
$14.00/1mg
Yield:-
Steps:
Multi-step reaction with 15 steps
1.1: 87 percent / hydroxylamine hydrochloride; NaOH / ethanol; H2O / 3 h / Heating
2.1: 88 percent / LiAlH4; diisopropylamine / tetrahydrofuran / 4 h / Heating
3.1: 85 percent / triethylamine / tetrahydrofuran / 3 h / 25 °C
4.1: 76 percent / Cs2CO3 / dioxane / 18 h / Heating
5.1: 75 percent / Cs2CO3 / acetonitrile / 20 h / 25 °C
6.1: AgOTf / CH2Cl2 / 1 h / 25 °C
6.2: 77 percent / TBAT / CH2Cl2 / 20 h / -45 - 25 °C
7.1: 74 percent / samarium diiodide; HMPA / 2-methyl-propan-2-ol / 3 h / -78 - -45 °C
8.1: 99 percent / Schwartz's reagent / tetrahydrofuran / 16 h / Heating
9.1: 86 percent / KHMDS / tetrahydrofuran / 1 h / 0 °C
10.1: 99 percent / HCl; methanol / 16 h / 25 °C
11.1: Yb(OTf)3*xH2O / CH2Cl2 / 48 h / 0 °C
11.2: 50 percent / ortho-iodoxybenzoic acid / dimethylsulfoxide / 20 h / 25 °C
12.1: 42 percent / chromium(II) chloride / acetone; H2O / 1 h / 25 °C
13.1: 99 percent / hydrogen / Pd/C / ethyl acetate / 6 h / 25 °C
14.1: 55 percent / pTsOH*H2O / CH2Cl2 / 7 h / 0 - 25 °C
15.1: 95 percent / NaBH4; MeOH / 1.17 h / -78 - 25 °C
References:
Eckelbarger, Joseph D.;Wilmot, Jeremy T.;Gin, David Y. [Journal of the American Chemical Society,2006,vol. 128,# 32,p. 10370 - 10371]

38750-57-1
1 suppliers
inquiry

24316-19-6
204 suppliers
$14.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24316-19-6
204 suppliers
$14.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24316-19-6
204 suppliers
$14.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24316-19-6
204 suppliers
$14.00/1mg