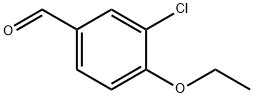
CHEMBRDG-BB 4015100 synthesis
- Product Name:CHEMBRDG-BB 4015100
- CAS Number:99585-10-1
- Molecular formula:C9H9ClO2
- Molecular Weight:184.62
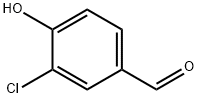
2420-16-8
222 suppliers
$5.00/1g

75-03-6
369 suppliers
$11.00/5g

99585-10-1
35 suppliers
$18.00/250mg
Yield:99585-10-1 98%
Steps:
R.5 3-Chloro-4-ethoxybenzaldehyde
REFERENCE EXAMPLE 5 3-Chloro-4-ethoxybenzaldehyde Following a similar procedure to that described in reference example 3, but starting from 3-chloro-4-hydroxybenzaldehyde instead of 4-hydroxybenzaldehyde and using ethyl iodide instead of 2-iodopropane, the title compound of the example was obtained as an oil (98% yield). 1H-NMR (300 MHz, CDCl3 δ TMS): 1.52 (t, J=6.9 Hz, 3H), 4.21 (q, J=6.9 Hz, 2H), 7.02 (d, J=8.7 Hz, 1H), 7.76 (d, J=8.7 Hz, 1H), 7.91 (s, 1H), 9.85 (s, 1H).
References:
US2003/176481,2003,A1
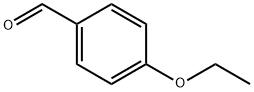
10031-82-0
294 suppliers
$6.00/5g

99585-10-1
35 suppliers
$18.00/250mg
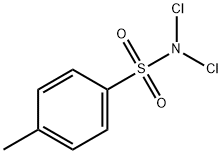
473-34-7
87 suppliers
$45.00/250mg

10031-82-0
294 suppliers
$6.00/5g

99585-10-1
35 suppliers
$18.00/250mg