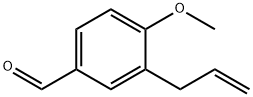
CHEMBRDG-BB 6564340 synthesis
- Product Name:CHEMBRDG-BB 6564340
- CAS Number:67483-48-1
- Molecular formula:C11H12O2
- Molecular Weight:176.21
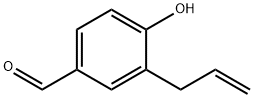
41052-88-4
15 suppliers
$90.00/100mg

74-88-4
360 suppliers
$15.00/10g

67483-48-1
22 suppliers
$45.00/50mg
Yield:67483-48-1 100%
Reaction Conditions:
with potassium carbonate in acetone at 20; for 3 h;
Steps:
5.1.7 Synthesis of 3-allyl-4-methoxybenzaldehyde 12b
A solution of 3-allyl-4-hydroxybenzaldehyde 11 (6.10g, 37.6mmol) in dry acetone (70mL) was added K2CO3 (9.73g, 70.1mmol) portionwise, followed by MeI (1.8mL, 38.0mmol) at room temperature. After 3h, the reaction mixture was then filtered and the filtercake was washed with acetone (100mL). The filtrate was concentrated under reduced pressure and the residue was dissolved in EtOAc (50mL), washed with water and brine. The organic layer was dried over MgSO4, and filtered and the filtrate was concentrated in vacuo to give 3-allyl-4-methoxybenzaldehyde 12b as a white crystalline solid.
References:
Liu, Zhiguo;Tang, Longguang;Zou, Peng;Zhang, Yali;Wang, Zhe;Fang, Qilu;Jiang, Lili;Chen, Gaozhi;Xu, Zheng;Zhang, Huajie;Liang, Guang [European Journal of Medicinal Chemistry,2014,vol. 74,p. 671 - 682]
40663-68-1
124 suppliers
$10.00/1g

67483-48-1
22 suppliers
$45.00/50mg
123-08-0
964 suppliers
$5.00/10g

67483-48-1
22 suppliers
$45.00/50mg
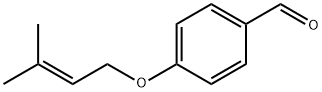
28090-12-2
90 suppliers
inquiry

67483-48-1
22 suppliers
$45.00/50mg