CHEMBRDG-BB 9071407 synthesis
- Product Name:CHEMBRDG-BB 9071407
- CAS Number:85582-62-3
- Molecular formula:C9H11FOS
- Molecular Weight:186.25
Yield:85582-62-3 96%
Reaction Conditions:
with potassium carbonate in acetone at 20; for 48 h;
Steps:
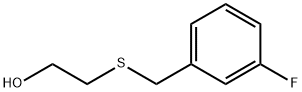
2-[(3-Fluorobenzyl)sulfanyl]ethanol
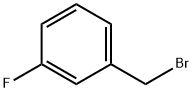
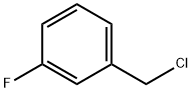
2-[(3-Fluorobenzyl)sulfanyl]ethanol 1 -(Bromomethyl)-3-fluorobenzene (1 .00 g, 5.29 mmol) was dissolved in acetone (10 mL) to which 2-sulfanylethanol (0.413 g, 5.29 mmol) was added followed by K2C03 (1 .097 g, 7.94 mmol, 1 .5 molar equivalent). The reaction mixture was stirred at room temperature for 48h. The solvent was removed under reduced pressure and the crude material was extracted with DCM and water. The organic layer was collected, dried (MgS04), filtered and the solvent removed under reduced pressure to give the required product as a pale yellow oil (0.945 g, 96%). IR (NaCI): 3395, 1616, 1589, 1485, 1446, 1267, 1 138, 1061 , 1013, 942, 884, 787cm 1 1 H NMR (DMSO-d6): 7.16-7.13(1 H, m), 7.08-7.03(3H, m), 4.77(1 1-1, t, J = 5.5Hz), 3.75(21-1, s), 3.51 -3.46(21-1, m), 2.48(21-1, t, J= 6.9Hz). HRESIMS: Found: 187.05874 calculated for C9H12OF32S 187.05874. Reference 1 : Czobor, Francisc; Cristescu, Carol. as-Triazine derivatives with potential therapeutic action. Part XXIX. Synthesis of 5-[2-(halogenobenzylthio)-ethyl]thio-6- azauracil derivatives as potential antiviral agents. Revue Roumaine de Chimie (2002), Volume Date 2001 , 46(9), 1007-101 1 . Reference 2: Kuliev, A. M.; Sultanov, Yu. M.; Aliev, I. A.; Mekhraliev, N. A. Synthesis of different derivatives of m-fluorobenzyl mercaptan. Katalit. Prevrashcheniya Organ. Soedin., Baku (1981 ), 51 -55.
References:
WO2014/23934,2014,A1 Location in patent:Page/Page column 18-19