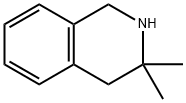
CHEMBRDG-BB 9071824 synthesis
- Product Name:CHEMBRDG-BB 9071824
- CAS Number:28459-83-8
- Molecular formula:C11H15N
- Molecular Weight:161.24
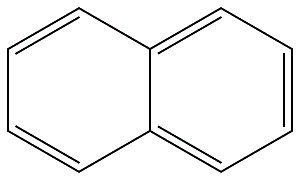
91-20-3
530 suppliers
$14.00/25g
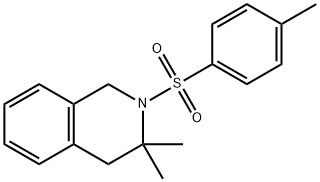
200631-40-9
5 suppliers
inquiry

28459-83-8
13 suppliers
$505.05/5MG
Yield:28459-83-8 86%
Reaction Conditions:
Stage #1: naphthalenewith sodium hydride in formaldehyde diethyl acetal; for 4 h;
Stage #2: 3,3-dimethyl-2-tosyl-1,2,3,4-tetrahydroisoquinoline in formaldehyde diethyl acetal; for 2 h;
Steps:
4 Example 4
Example 4 To a stirred solution of naphthalene (5.8 g, 0.045 mol) in diethoxymethane (50 ML) was added sodium metal (1.09 g, 0.039 mol.).. The mixture was allowed to stir for four (4) hours until a dark green color persisted.. To this was added of N-toluenesulfonyl-3,3-dimethyl-1,2,3,4-tetrahydroisoquinoline (5.0 g, 0.016 mol.) in 20 ML of dimethoxymethane.. The reaction was monitored by gas chromatography.. When the reaction was complete (2 hours), the mixture was quenched with saturated sodium chloride (70 ML).. The mixture was partitioned between ethyl acetate (250 ML) and 10% HCL (250 ML) and the organic layer was discarded. 10% sodium hydroxide was added to the aqueous layer until a PH=7 was obtained.. The aqueous layer was further extracted over methylene chloride (2 x 100 ML), dried over magnesium sulfate, filtered and concentrated (25°C/150 torr) to produce 2.2 g (86%) of 3,3-dimethyl-1,2,3,4-tetrahydroisoquinoline. IR (neat, cm-1) 3043, 2897, 744;1H-NMR (300 Mhz, CDCl3) δ 7.14-7.00 (m, 4H), 4.02 (s, 2H), 2.61 (s, 2H), 1.58 (bs, 1H), 1.19 (s, 6H);13C-NMR (75 Mhz, CDCl3) 134.5, 134.4, 129.5, 125.9, 125.6, 125.5, 48.6, 44.3, 41.5, 27.7; MS m/z (M+) calc'd 161.24, observed 161.
References:
EP929527,2004,B1 Location in patent:Page 5
1197-21-3
0 suppliers
$18.00/1mg

28459-83-8
13 suppliers
$505.05/5MG
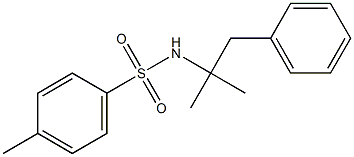
110871-36-8
3 suppliers
inquiry

28459-83-8
13 suppliers
$505.05/5MG

200631-40-9
5 suppliers
inquiry

28459-83-8
13 suppliers
$505.05/5MG
