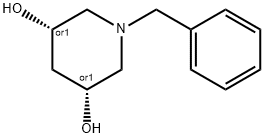
cis-1-Benzyl-piperidine-3,5-diol synthesis
- Product Name:cis-1-Benzyl-piperidine-3,5-diol
- CAS Number:913831-96-6
- Molecular formula:C12H17NO2
- Molecular Weight:207.27
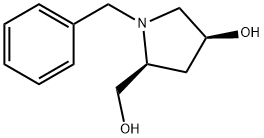
635299-92-2
4 suppliers
inquiry

913831-96-6
1 suppliers
inquiry
Yield:913831-96-6 91.7%
Reaction Conditions:
Stage #1: (3S,5S)-1-benzyl-5-hydroxymethylpyrrolidin-3-olwith trifluoroacetic anhydride in tetrahydrofuran at 0; for 1 h;
Stage #2: with triethylamine in tetrahydrofuran;Reflux;
Stage #3: with sodium hydroxide in tetrahydrofuran at 0; for 2 h;
Steps:
XL.4 Step 4: l-Benzyl-piperidine-3,5-diol
Step 4: l-Benzyl-piperidine-3,5-diol To the solution of l-benzyl-5-hydroxymethyl-pyrrolidin-3-ol (0.6 g, 2.9 mmol) in THF (20 mL) was added TFAA (1.06 mL, 7.24 mmol) dropwise at 0 °C, after the mixture was stirred for 1 hour later, Et3N (0.88 g, 8.69 mmol) was added, then the mixture was heated to reflux overnight. After the mixture was cooling to 0 °C, 3.75 M NaOH solution was added and stirred for 2 hours. The mixture was washed with IN HC1 solution and extracted with EtOAc (30 mL x 2). The combined organic phase was dried over Na2S04, filtered and the filtrate was concentrated under vacuum to give the crude product which was purified by column to give l-benzyl-piperidine-3,5-diol (0.55 g, 91.7% yield): 1H NMR (400 MHz, CD3OD) δ 7.37-7.23 (m, 5H), 4.21-4.20 (m, 1H), 4.10-4.09 (m, 1H), 3.62-3.60 (m, 1H), 2.90-2.89 (m, 2H), 2.50-2.47 (m, 2H), 2.39-2.32 (m, 1H), 1.81-1.80 (m, 1H), 1.72-1.65 (m, 1H); ES-LCMS m/z 208 (M+H).
References:
WO2013/149362,2013,A1 Location in patent:Page/Page column 63-64
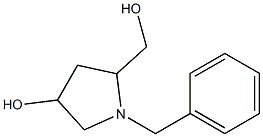
635299-91-1
2 suppliers
inquiry

913831-96-6
1 suppliers
inquiry

1792-78-5
1 suppliers
inquiry

913831-96-6
1 suppliers
inquiry
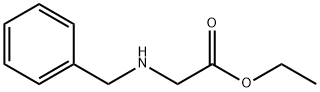
6436-90-4
374 suppliers
$8.00/10g

913831-96-6
1 suppliers
inquiry

15057-40-6
6 suppliers
inquiry

913831-96-6
1 suppliers
inquiry