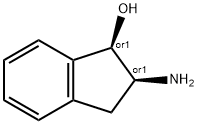
Cis-2-Amino-1-hydroxyindane synthesis
- Product Name:Cis-2-Amino-1-hydroxyindane
- CAS Number:23337-80-6
- Molecular formula:C9H11NO
- Molecular Weight:149.19
15028-10-1
58 suppliers
$45.00/250mg

23337-80-6
9 suppliers
$267.00/100mg
Yield:23337-80-6 97%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 25; under 2327.23 Torr; for 8 h;diastereoselective reaction;Reagent/catalyst;
Steps:
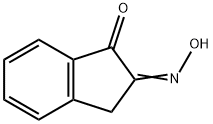
General procedures for the synthesis of (±)-cis-2-amino-1-indanol (3) and(±)-trans-1-amino-2-indanol (19)
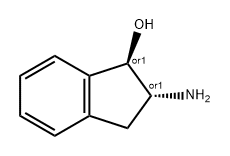
General procedure: General synthetic procedures for 3 (10% Pd/C 500 mg, 25 °C condition) and 19 (10% Pd/BaSO4 500 mg, 25 °C condition): To a solution of 1,2-indanedion-2-oxime (2, 1g, 6.2 mmol for synthesis of 3) or 1,2-indanedion-1-oxime (15, 1 g, 6.2 mmol for synthesis of 19) in absolute ethanol (30 mL) was added 10% Pd-catalyst (500 mg). The reaction mixture was shaken in the Parr hydrogenation apparatus for 8 h under 45 psi of hydrogen gas. The reaction mixture was filtered on celite pad and washed with cold ethanol and concentrated under reduced pressure to give the crude residue. The residue was purified by column chromatography using MeOH/CH2Cl2 (1:18, 1:9, or 1:4) to afford the diastereoselectively pure cis-2-amino-1-indanol (3) and trans-1-amino-2-indanol(19), respectively.
References:
Nguyen, Thi Ha;Ma, Eunsook [Synthetic Communications,2021,vol. 51,# 24,p. 3717 - 3728] Location in patent:supporting information

15028-10-1
58 suppliers
$45.00/250mg

23337-80-6
9 suppliers
$267.00/100mg
13575-72-9
18 suppliers
$105.00/25mg

13943-70-9
0 suppliers
inquiry

23337-80-6
9 suppliers
$267.00/100mg
95-13-6
316 suppliers
$6.00/25g

23337-80-6
9 suppliers
$267.00/100mg
![Indeno[1,2-b]azirine, 1,1a,6,6a-tetrahydro-](/CAS/20210305/GIF/26536-31-2.gif)
26536-31-2
0 suppliers
inquiry

23337-80-6
9 suppliers
$267.00/100mg

13575-72-9
18 suppliers
$105.00/25mg