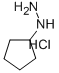
Cyclopentylhydrazine hydrochloride synthesis
- Product Name:Cyclopentylhydrazine hydrochloride
- CAS Number:24214-72-0
- Molecular formula:C5H13ClN2
- Molecular Weight:136.62
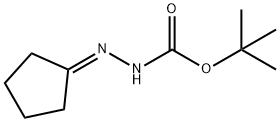
79201-39-1

24214-72-0
Step 2: Synthesis of cyclopentylhydrazine hydrochloride Tert-butyl N'-cyclopentylidenehydrazine formate (5.448 g, 27.48 mmol) was dissolved in a solvent mixture of anhydrous tetrahydrofuran (25 mL) and anhydrous methanol (34 mL). Under argon protection, sodium cyanoborohydride (2.044 g, 32.53 mmol) was added in batches, followed by refluxing the reaction mixture for 10 min. Upon completion of the reaction, it was cooled to room temperature, 6N hydrochloric acid (12 mL) was added, and refluxing was continued for 1.5 hours. An aliquot was analyzed by NMR and it was confirmed that some of the BOC protecting groups were still not removed. Add 6N hydrochloric acid and continue refluxing for 3 hours. At the end of the reaction, cooled to room temperature and stirred overnight. The insoluble material was removed by filtration, and the filtrate was concentrated and azeotropized with toluene three times to completely remove the water. The residue was dissolved in hot isopropanol, cooled to room temperature, diluted with ether and further cooled. The solid was collected by filtration to afford cyclopentylhydrazine hydrochloride (3.903 g, 103% yield), which could be used in subsequent reactions without further purification.

79201-39-1
30 suppliers
$45.00/25mg

24214-72-0
124 suppliers
inquiry
Yield:24214-72-0 100%
Reaction Conditions:
Stage #1: tert-butyl 2-cyclopentylidenehydrazine carboxylatewith sodium cyanoborohydride in tetrahydrofuran;methanol; for 0.166667 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water at 20; for 4.5 h;Heating / reflux;
Steps:
64.2
Step 2: Cyclopentyl-hydrazine hydrochloride N'-Cyclopentyl-hydrazinecarboxylic acid tert-butyl ester (5.448 g, 27.48 mmol) was dissolved in dry tetrahydrofuran (25 mL) and dry methanol (34 mL). Sodium cyanoborohydride (2.044 g, 32.53 mmol) was added portionwise and then the mixture was refluxed under argon for 10 minutes. After cooling to room temperature, 6N HCl (12 mL) was added and the mixture was refluxed for 1.5 hours. The NMR of an aliquot at this time showed some BOC-protected material to still be present. Additional 6N HCl was added and the mixture was refluxed for another 3 hours and then cooled to room temperature and stirred overnight. The mixture was filtered to remove the insoluble material, concentrated and azeotroped three times with toluene to remove the water. The residue was dissolved in hot isopropanol, cooled to room temperature, diluted with ether and chilled. Filtration yielded cyclopentyl-hydrazine hydrochloride (3.903 g, 103%) which was used without any further purification.
References:
US2007/225280,2007,A1 Location in patent:Page/Page column 48
646071-31-0
28 suppliers
inquiry

24214-72-0
124 suppliers
inquiry
259870-74-1
3 suppliers
inquiry

24214-72-0
124 suppliers
inquiry
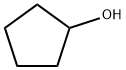
96-41-3
266 suppliers
$10.00/10g

24214-72-0
124 suppliers
inquiry

287729-05-9
0 suppliers
inquiry

24214-72-0
124 suppliers
inquiry