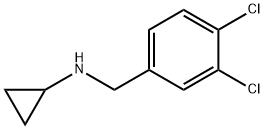
Cyclopropyl-(3,4-dichloro-benzyl)-aMine synthesis
- Product Name:Cyclopropyl-(3,4-dichloro-benzyl)-aMine
- CAS Number:90919-75-8
- Molecular formula:C10H11Cl2N
- Molecular Weight:216.11
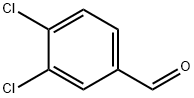
6287-38-3
259 suppliers
$8.00/10g
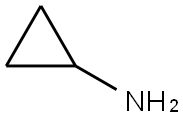
765-30-0
507 suppliers
$10.00/25g

90919-75-8
14 suppliers
$349.00/500mg
Yield:90919-75-8 62%
Reaction Conditions:
Stage #1: 3,4-dichlorobenzaldehyde;Cyclopropylamine in 1,2-dichloro-ethane; for 3 h;Inert atmosphere;
Stage #2: with sodium tris(acetoxy)borohydride in 1,2-dichloro-ethane at 20; for 18 h;Inert atmosphere;
Steps:
N-(3,4-dichlorobenzyl)cyclopropanamine
3,4-dichlorobenzaldehyde (300 mg, 1.71 mmol, 1.0 eq) and the cyclopropylamine (0.178 ml, 2.57 mmol, 1.5 eq) were combined in anhydrous 1,2-dichloroethane (6 ml) under an atmosphere of nitrogen and stirred for 3 hours. Sodium triacetoxyborohydride (509 mg, 2.40 mmol, 1.4 eq) was added and the reaction was stirred at room temperature for 18 hours. The reaction mixture was poured into 2M aqueous sodium carbonate and extracted with ethyl acetate (3× 10mL). The combined organic extracts were dried (MgSO4), concentrated under reduced pressure and purified by column chromatography on silica eluting with a stepped gradient of 2N NH3 in methanol: ethyl acetate: hexane (1:10:89 → 1:30:69 → 1:50:49) to yield the product as a pale yellow oil (227 mg, 62%).
References:
Brear, Paul;North, Andrew;Iegre, Jessica;Hadje Georgiou, Kathy;Lubin, Alexandra;Carro, Laura;Green, William;Sore, Hannah F.;Hyv?nen, Marko;Spring, David R. [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 11,p. 3016 - 3020] Location in patent:supporting information