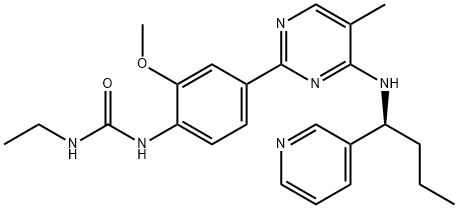
CYT997 synthesis
- Product Name:CYT997
- CAS Number:917111-44-5
- Molecular formula:C24H30N6O2
- Molecular Weight:434.53
![4-Pyrimidinamine, 2-chloro-5-methyl-N-[(1S)-1-(3-pyridinyl)butyl]-](/CAS/20210305/GIF/854074-39-8.gif)
854074-39-8
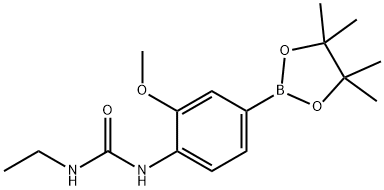
917111-46-7

917111-44-5
Under nitrogen protection, 2-chloro-5-methyl-N-[(1S)-1-pyridin-3-ylbutyl]pyrimidin-4-amine (277 mg, 1.0 mmol), 4-{[(ethylamino)carbonyl]amino}-3-methoxybenzeneboronic acid pinacol ester (416 mg, 1.3 mmol), and tetrakis(triphenylphosphine)palladium(0) (116 mg, 0.1 mmol) were were dissolved in a mixture of toluene-n-propanol (12 mL, 3:1, v/v) and 2M aqueous sodium carbonate (750 μL, 1.5 mmol) was added. The reaction mixture was heated at 100 °C for 17 h with vigorous stirring. After the reaction was completed, it was cooled to room temperature, diluted by adding ethyl acetate (25 mL), and the mixture was washed sequentially with water (6 × 15 mL), brine (20 mL), and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to obtain the crude product. The crude product was purified by column chromatography using dichloromethane-methanol-ammonia (93:7:1, v/v/v) as eluent to give the target product (110 mg, 57% yield).1H NMR (CDCl3) δ 0.99 (t, 3H, J = 7.4 Hz, CH3), 1.18 (t, 3H, J = 7.2 Hz, CH3), 1.33- 1.60 (m, 2H, CH2), 1.82-2.02 (m, 2H, CH2), 2.11 (s, 3H, Ar-Me), 3.25-3.39 (m, 2H, CH2), 3.87 (s, 3H, OMe), 4.80-4.96 (m, 2H, 2 × NH), 5.26-5.36 (m, 1H, CH), 6.98 (br s, 1H, CH), 4.80-4.96 (m, 1H, CH), 5.26-5.36 (m, 1H, CH), 6.98 (br s, 1H, J = 7.2 Hz, CH 6.98 (br s, 1H, Ar-NHCONH), 7.22-7.28 (m, 1H, ArH), 7.67-7.72 (m, 2H, ArH), 7.83-7.88 (m, 1H, ArH), 8.05 (d, 1H, J = 0.8 Hz, ArH), 8.10 (d, 1H, J = 8.2 Hz, ArH), 8.47 (dd, 1H, J = 8.2 Hz, ArH), 8.47 (dd, 1H, J = 8.2 Hz, ArH), 8.47 (dd, 1H, J = 8.2 Hz, ArH) 8.47 (dd, 1H, J = 6.6, 1.8 Hz, ArH), 8.69 (d, 1H, J = 2.0 Hz, ArH).
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

917111-46-7
37 suppliers
$55.00/25mg

917111-44-5
97 suppliers
$35.00/1mg
Yield: 60%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in propan-1-ol;water;toluene at 100; for 44 h;Inert atmosphere;
References:
Burns, Christopher J.;Harte, Michael F.;Bu, Xianyong;Fantino, Emmanuelle;Joffe, Max;Sikanyika, Harrison;Su, Stephen;Tranberg, C. Elisabet;Wilson, Neil;Charman, Susan A.;Shackleford, David M.;Wilks, Andrew F. [Bioorganic and Medicinal Chemistry Letters,2009,vol. 19,# 16,p. 4639 - 4642] Location in patent:scheme or table