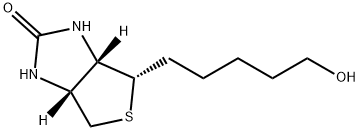
D-BIOTINOL synthesis
- Product Name:D-BIOTINOL
- CAS Number:53906-36-8
- Molecular formula:C10H18N2O2S
- Molecular Weight:230.33
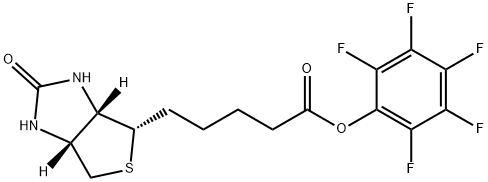
120550-35-8
121 suppliers
$60.00/25 mg

53906-36-8
24 suppliers
inquiry
Yield: 94%
Reaction Conditions:
with sodium tetrahydroborate in N,N-dimethyl-formamide at 0; for 0.25 h;
Steps:
Synthesis of N6-(((5-((3aS,4S,6aR)-2-oxohexahydro-lH-thieno[3,4- d]imidazol-4-yl)pentyl)oxy)carbonyl)-L-lysine (1):
D-Biotin (1.00 g, 4.09 mmol) was dissolved in 20 mL DMF at 70° C and allowed to cool to rt. TEA (0.83g, 1.14 mL, 8.19 mmol) was added, followed by pentafluorophenyl trifluoroacetate (1.60 g, 0.98 mL, 5.73 mmol). The reaction was allowed to stir for 1 h at 0° C and became pink. Solvent was reduced to 1 mL in vacuo and the crude material was triturated with cold diethyl ether. The pFp ester product was recovered as a white solid (1.54 g, 96%). Biotin-pFp ester (1.00 g, 2.44 mmol) was dissolved in 10 mL DMF and cooled to 0° C. A flask containing a suspension of NaBH4 (3.4 mmol) in dry DMF (5 mL) was also cooled to 0 °C. The pFp-ester was transferred dropwise via a cannula over 15 min and the mixture was stirred at 0 °C. The reaction was followed by TLC and upon completion the cold mixture was acidified with 1 N HC1 and reduced to 1 mL in vacuo. The residue was triturated with cold diethyl ether and the product alcohol was recovered as a white solid (0.528 g, 94%). The alcohol (0.528 g, 2.29 mmol) and TEA (0.39 ml, 1.2 eq) were dissolved in DMF (10 mL) and added dropwise to a stirred solution of 4-nitrophenyl chloroformate (4-NCF, 1.38 g, 6.87 mmol, 3.0 eq) in DMF (10 mL) over a period of 1 h at -10°C. The reaction mixture was allowed to warm to rt, stirred overnight, and subsequently and reduced to 1 mL in vacuo. The residue was triturated with cold diethyl ether. The nitrophenol carbonate product was recovered as a white solid (0.96 g, 95%). Fmoc-L-Lys-OH (1.03 g, 2.83 mmol, 1.3 eq.) was suspended under argon in anhydrous DMF (10 ml) containing DiPEA (0.50 ml, 1.3 eq.). To this white suspension, a clear solution of the nitrophenol-carbonate (2.18 mmol, 1.0 eq.) in anhydrous DMF (10 mL) was added drop wise under argon at rt over a period of 2 h. The reaction mixture was stirred for additional 4 h at rt, before the solution as acidified to pH 2 with 1 N HC1. All volatiles were evaporated under reduced pressure and the residue was triturated with cold diethyl ether. The crude product was purified by column chromatography (DCM : MeOH 95 : 5 v/v) to give the Fmoc-protected ncAA as a white solid. The Fmoc- protected ncAA was dissolved in 20% piperidine in DMF (5 ml) and stirred for 1 h at r.t.. All volatiles were removed under reduced pressure and the residue was triturated with cold diethyl ether. Drying of the residue in vaccuum yield the pure ncAA as a white powder (0.622 g, 71 %). 1H-NMR (D6-DMSO, 400 MHz): δ = 1.30-1.45 (m, 6H), 1.50-1.65 (m, 4H), 1.85 (m, 2H), 2.65 (d, J = 12.2 Hz, 1H), 2.84 (dd, J= 5.0 Hz, J= 12.2 Hz, 1H), 2.95 (m, 2H), 3.13 (m, 1H), 3.42 (m, 1H), 3.66 (m, 1H), 3.85 (dd, J= 13.8 Hz, J = 1.0 Hz 1H), 3.93 (t, J= 7.5 Hz, 2H), 4.17 (dd, J= 8.2 Hz, J= 7.3 Hz, 1H), 4.35 (dd, J= 8.1 Hz, J= 7.2 Hz, 1H), 7.11 (t, J= 5.4 Hz, 1H), 8.50 (br, 3H), 8.53 (br, 1H), 8.68 (br, 1H).13C-NMR (D6-DMSO, 100 MHz): 13C-NMR (CDCI3, 100 MHz): δ = 171.44, 163.29, 156.82, 64.00, 61.61, 60.35, 59.79, 55.95, 52.28, 39.10, 30.05, 29.35, 29.02, 28.78, 28,73, 25.91, 22.05. HR-MS (C17H31N4O5S): calculated: 403.20097, found: 403.20159.
References:
KING ABDULLAH UNIVERSITY OF SCIENCE AND TECHNOLOGY;HOHL, Adrian;EPPINGER, Jörg WO2018/207077, 2018, A1 Location in patent:Paragraph 0082
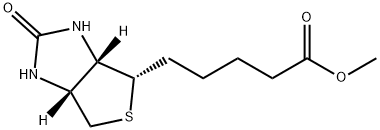
608-16-2
53 suppliers
$35.00/100mg

53906-36-8
24 suppliers
inquiry
![ethyl [3aS-(3aalpha,4beta,6aalpha)]-hexahydro-2-oxo-1H-thieno[3,4-d]imidazole-4-valerate](/CAS/GIF/87573-52-2.gif)
87573-52-2
9 suppliers
inquiry

53906-36-8
24 suppliers
inquiry
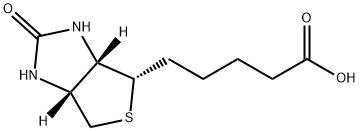
58-85-5
1050 suppliers
$5.00/10mg

53906-36-8
24 suppliers
inquiry
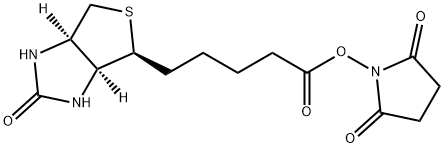
35013-72-0
394 suppliers
$12.00/100mg

53906-36-8
24 suppliers
inquiry