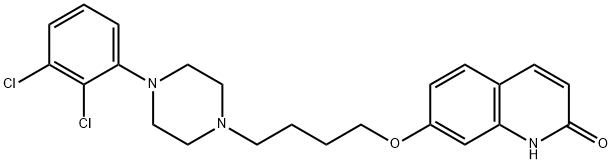
DEHYDRO ARIPIPRAZOLE synthesis
- Product Name:DEHYDRO ARIPIPRAZOLE
- CAS Number:129722-25-4
- Molecular formula:C23H25Cl2N3O2
- Molecular Weight:446.37
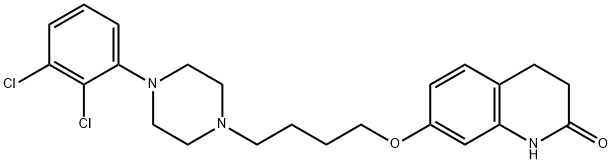
129722-12-9

129722-25-4
The general procedure for the synthesis of 7-[4-[4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy]-3,4-dihydroquinolin-2(1H)-ones from 7-(4-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-2(1H)-quinolinone was as follows: Example 1: Large-scale synthesis of dehydroaripiprazole [7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)quinolin-2(1H)-one] (Compound 1; Formula VI) Aripiprazole (Formula VIA) 1. 15 g (0.03 mol) of 7-(4-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one was dissolved in 360 mL of anhydrous tetrahydrofuran to form a clarified solution. 2. 12 mL (0.16 mol) of trifluoroacetic acid was added to the solution. 3. 24.3 g (0.11 mol) of 1,2-dichloro-4,5-dicyanoquinone was added and the mixture was stirred at room temperature under a nitrogen atmosphere. 4. After the reaction was stirred for 40 minutes, TLC assay showed complete consumption of the raw material. 5. 1.5 L of water was added and then the reaction mixture was alkalized to pH 12 with 50% NaOH aqueous solution. 6. The reaction mixture was extracted with dichloromethane (3 x 300 mL), the organic phases were combined and dried with MgSO4. 7. The crude product was purified by column chromatography using silica as stationary phase and dichloromethane to 10% methanol/dichloromethane as eluent. 8. The product was purified by 2-propanolysis. 8. The product was further purified by recrystallization from 2-propanol to give 13.7 g (92%) of off-white solid target product. Product characterization: 1H-NMR (400 MHz, CDCl3) δ 12.33 (1H, br s), 7.72 (1H, d), 7.42 (1H, d), 7.16-7.11 (2H, m), 6.98-6.93 (1H, dd), 6.83-6.79 (2H, m), 6.53 (1H, d), 4.10 (2H, t), 3.09 (4H, br), 4.10 (2H, t), 4.10 (2H, t). 3.09 (4H, br s), 2.67 (4H, br s), 2.52 (2H, t), 1.93-1.70 (4H, m). LCMS (acidic method) [M + H]+ 446.02, rt 14.246 min.

129722-12-9
597 suppliers
$5.00/100mg

129722-25-4
151 suppliers
$45.00/100mg
Yield:129722-25-4 92%
Reaction Conditions:
Stage #1:aripiprazole with trifluoroacetic acid;2,3-dicyano-5,6-dichloro-p-benzoquinone in tetrahydrofuran at 20; for 0.666667 h;Inert atmosphere;large scale reaction;
Stage #2: with sodium hydroxide in tetrahydrofuran;water; pH=12
Steps:
1
Example 1 Large scale synthesis of dehydroaripiprazole [7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)quinolin-2(1H)-one] (Compound 1; Formula VI) Aripiprazole (Formula VIA; 15 g, 0.03 mol) was dissolved in anhydrous tetrahydrofuran (360 mL). Trifluoroacetic acid (12 mL, 0.16 mol) was added to the clear solution. 1,2-Dichloro-4,5-dicyano quinone (24.3 g, 0.11 mol) was added and the mixture was stirred at room temperature under nitrogen atmosphere. The reaction was stirred for 40 minutes and TLC showed no starting material remaining Water (1.5 L) was added then reaction mixture basified with 50% aq NaOH until pH 12. The reaction mixture was extracted with dichloromethane (3*μL) and the combined organics were dried (MgSO4) to give the crude product. This was purified by column chromatography on silica eluting with dichloromethane to 10% methanol/dichloromethane. The product was further purified by recrystallisation from 2-propanol to give the desired product (13.7 g, 92%) as an off white solid. 1H-NMR (400 MHz, CDCl3) δ 12.33 (1H, br s), 7.72 (1H, d), 7.42 (1H, d), 7.16-7.11 (2H, m), 6.98-6.93 (1H, dd), 6.83-6.79 (2H, m), 6.53 (1H, d), 4.10 (2H, t), 3.09 (4H, br s), 2.67 (4H, br s), 2.52 (2H, t), 1.93-1.70 (4H, m). LCMS (acidic method) [M+H]+ 446.02, rt 14.246 min.
References:
Alkermes, Inc. US2011/275803, 2011, A1 Location in patent:Page/Page column 60-61
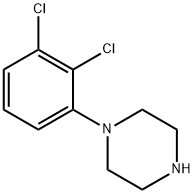
41202-77-1
249 suppliers
inquiry

203395-59-9
95 suppliers
$8.00/250mg

129722-25-4
151 suppliers
$45.00/100mg
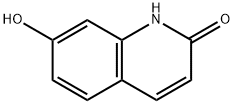
70500-72-0
435 suppliers
$6.00/250mg

129722-25-4
151 suppliers
$45.00/100mg