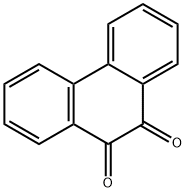
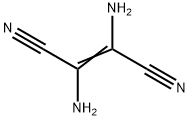
Dibenzo[f,h]quinoxaline-2,3-dicarbonitrile synthesis
- Product Name:Dibenzo[f,h]quinoxaline-2,3-dicarbonitrile
- CAS Number:55408-49-6
- Molecular formula:C18H8N4
- Molecular Weight:280.2829
Yield:55408-49-6 95 %
Reaction Conditions:
with calcium iodate in ethanol at 20;Catalytic behavior;Green chemistry;
Steps:
Typical procedure for preparation of compounds (3a-q)
General procedure: 1,2-Arylenediamine, 1a-d, or 2,3-diaminomaleonitrile, 1e, (0.1 mmol) and the corresponding1,2-diketones (0.1 mmol), 2a-d, were dissolved in ethanol by continuousstirring. A catalytic amount (3 mol%, 0.005 gr) of Ca(IO3)2 was added to thesolution. The reaction mixture was then stirred at room temperature for 3-20 min(Tables 2 and 3). The reaction progress was monitored by TLC. After completion ofthe reaction, the used catalyst was collected by filtration and cold water was addedto the filtrate to obtain the product. Afterward, the residue was filtered and washedwith cold ethanol/water to yield compounds 3a-q. In this method, pure productswere obtained. For further purifications, the crude products were recrystallized fromEtOH or MeOH/AcOH mixtures. .
References:
Kalhor, Mehdi;Shayestefar, Mahboubeh;Khalaj, Mehdi;Janghorban, Fatemeh [Research on Chemical Intermediates,2023,vol. 49,# 3,p. 885 - 900]