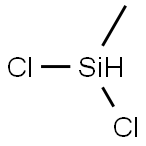
Dichloromethylsilane synthesis
- Product Name:Dichloromethylsilane
- CAS Number:75-54-7
- Molecular formula:CH4Cl2Si
- Molecular Weight:115.03
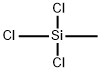
75-79-6
293 suppliers
$15.00/5 g
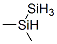
870-26-8
2 suppliers
inquiry

75-54-7
180 suppliers
$32.47/25G
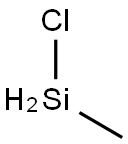
993-00-0
13 suppliers
inquiry
Yield:75-54-7 72.9 %Spectr. ,993-00-0 9.4 %Spectr.
Reaction Conditions:
with tetra-n-butylphosphonium chloride in diethylene glycol dimethyl ether at -196 - 120; for 19 h;Sealed tube;Temperature;
Steps:
2; 4 Example 1
General procedure: MeH2Si-SiH2Me (VIII, 0.8 mmol) and MeSiC (1.3 mmol) were mixed with a catalytic amount of the redistribution catalyst of n-Bu4PCI (0.02 mmol) in diglyme (0.35 ml) as solvent in an NMR tube, solidified at -196 °C (liquid nitrogen) and sealed in vacuo. After warming the samples to r.t.,29Si- and1H-NMR spectra were measured to prove the degree of SiH/SiCI redistributions after different reaction times and temperatures to control and quantify product formation by integration of the intensity of relevant NMR signals within the mixture. As displayed in Table 2 already after 14 h at 80 °C chlorosilane IV was nearly completely consumed to give nearly equimolar amounts of the target compounds V and VI (41 % vs. 42%). Taking the formation of hydridosilane VII (16%) into consideration, silicon-silicon bond cleavage, hydrogenation and subsequent redistribution reactions occurred quantitatively. Increasing the reaction temperature and time to 120 °C/42 h did not shift the redistribution equilibrium significantly. Table 2
References:
MOMENTIVE PERFORMANCE MATERIALS INC.;AUNER, Norbert;SANTOWSKI, Tobias;STURM, Alexander, G. WO2019/60486, 2019, A1 Location in patent:Page/Page column 41; 42

75-79-6
293 suppliers
$15.00/5 g

75-54-7
180 suppliers
$32.47/25G
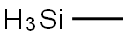
992-94-9
0 suppliers
$932.00/10g

993-00-0
13 suppliers
inquiry

75-79-6
293 suppliers
$15.00/5 g

75-54-7
180 suppliers
$32.47/25G