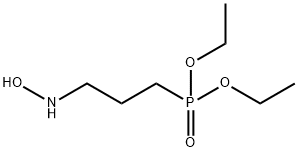
Diethyl 3-(N-Hydroxyamino)propylphosphate synthesis
- Product Name:Diethyl 3-(N-Hydroxyamino)propylphosphate
- CAS Number:66508-19-8
- Molecular formula:C7H18NO4P
- Molecular Weight:211.2
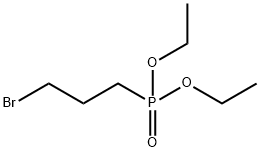
1186-10-3
95 suppliers
$21.00/1g

66508-19-8
13 suppliers
inquiry
Yield:66508-19-8 74.3%
Reaction Conditions:
Stage #1: diethyl 3-(bromopropyl)phosphonic acidwith sodium hydroxide;hydroxylamine hydrochloride in methanol;water at 40 - 45; for 3 h;
Stage #2: with sodium hydrogencarbonate in water; pH=8;
Steps:
5 EXAMPLE 5; Preparation of 3-(N-Hydroxyamino)-propylphosphonic Acid Diethylester
Firstly 32.0 g (0.8 mol) of sodium hydroxide dissolved in 75 ml water then 75 ml methanol, and finally 25.5 g (0.098 mol) 3-bromopropyl phosphonic acid diethylester were added drop by drop to a solution of 55.6 g (0.8 mol) hydroxylamine hydrochloride in 100 ml water with cooling with ice. This led to a clouding of the solution. After 3 hours stirring at a temperature of 40 to 45° C. methanol was removed under reduced pressure, the resulting aqueous solution was saturated with NaHCO3 (pH=8), shaken out three times with 60 ml toluene in each case (the toluene phase was discarded) and then shaken out with chloroform (1* with 90 ml, 2* with 50 ml in each case). The slightly yellowy chloroform phase was dried over MgSO4. After filtering of the dehydrating agent the solution was concentrated under reduced pressure. 15.43 g (0.0728 mol) 3-(N-hydroxyamino)-propylphosphonic acid diethylester were obtained as an almost colourless oil. This corresponds to a yield of 74.3% (DE-A-27 33 658). 1H-NMR (CDCl3) δ=5.94 (wide s, 2H), 4.13 (quintet, J=7 Hz, 4H) 2.90 (t, J=7 Hz, 2H) 1.5-2.2 (m, 4H), 1.33 (t, J=7 Hz, 6H); 13C-NMR (CDCl3) δ=61.23 (OCH2CH3), 53.34 (NCH2, J=15, 9 Hz), 22.75 (J=141, 9 Hz), 19.77 Hz, 16.08 (OCH2CH3).
References:
US6680308,2004,B1 Location in patent:Page column 14