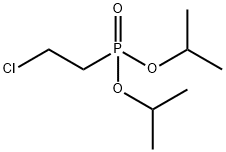
DIISOPROPYL-(2-CHLOROETHYL)-PHOSPHONATE synthesis
- Product Name:DIISOPROPYL-(2-CHLOROETHYL)-PHOSPHONATE
- CAS Number:25131-74-2
- Molecular formula:C8H18ClO3P
- Molecular Weight:228.65
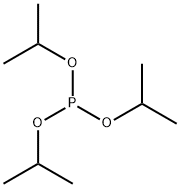
116-17-6
190 suppliers
$14.00/5mL

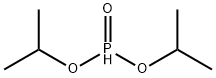
107-06-2
439 suppliers
$14.00/25g
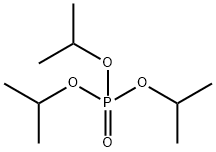
1809-20-7
185 suppliers
$8.00/10g

25131-74-2
10 suppliers
inquiry
Yield:25131-74-2 89.2 %Chromat. ,1809-20-7 8.5 %Chromat.
Reaction Conditions:
at 160; for 3.16667 h;Product distribution / selectivity;Microwave irradiation;Inert atmosphere;Michaelis-Arbuzov Reaction;
Steps:
1.20
Example 1; The optimization of the preparation of diisopropyl 2-chloroethylphosphonate (1) by the reaction of 1,2-dichloroethane (A) with triisopropylphosphite (B) with the simultaneous production of diisopropyl phosphonate (C), triisopropyl phosphate (D) and tetraisopropyl ethylenebisphosphonate (E) as by-products.; For the optimization of this reaction, a Type I MW reactor was used (with reaction test tubes of a volume of 10 ml). Triisopropylphosphite (2.0 ml; 8 mmol) and the corresponding amount of 1,2-dichloroethane were put into the MW test tubes (see Table 1). Entry 20 was prepared from half of the amount so that the 10 ml test tube would not be overfilled - 1.0 ml of triisopropylphosphite (4 mmol) and 2.7 ml of 1 ,2-dichloroethane (40 mmol). Because of the low amount of 1,2-dichloroethane, Entry 30 was prepared from a doubled amount - 4.0 ml of triisopropylphosphite (16 mmol) and 1.3 ml of 1,2-dichloroethane (19 mmol). The reaction test tubes were always securated five times and subsequently MW-heated following Table 1. Table 1. Conditions of the optimization of the preparation of diisopropyl 2- chloroethylphosphonate (1) following Example 1; Note: a (without securation), b (+20 molar equivalents of toluene), c (securated 5x), d (securated 5x, opened for air access and closed again), e (entry 15 after the reaction, opened for air access, closed, and heated for another 80 min.), / (+1 molar equivalent of toluene), g (+10 molar equivalents of /^-xylene), h (+10 molar equivalents of tetrahydrofurane - THF), i (+10 molar equivalents of pyridine), (+10 molar equivalents of diglyme), k (+10 molar equivalents of CH3CN), / (+10 molar equivalents of N,N-dimethylformamide - DMF). The influence of the addition of organic solvent was studied for the needs of a continuous-flow MW reactor, where the pressure impermeability of the whole system was subsequently tested by a suitable solvent (one not reducing the yield of the conducted MW reaction - i.e. CH3CN or DMF), which was released from the reactor before the attachment of the reaction mixture. The pressure impermeability of the reactor thus does not have to be tested by a relatively dangerous and toxic reaction mixture, but only by a suitable solvent whose residue in the MW reactor does not reduce the yield of the reaction at all.
References:
WO2012/13168,2012,A1 Location in patent:Page/Page column 11-13

116-17-6
190 suppliers
$14.00/5mL

107-06-2
439 suppliers
$14.00/25g

1809-20-7
185 suppliers
$8.00/10g
513-02-0
42 suppliers
$52.10/100g

25131-74-2
10 suppliers
inquiry

116-17-6
190 suppliers
$14.00/5mL

107-06-2
439 suppliers
$14.00/25g

1809-20-7
185 suppliers
$8.00/10g

513-02-0
42 suppliers
$52.10/100g
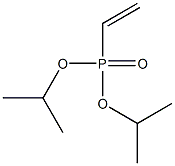
4472-27-9
2 suppliers
inquiry

25131-74-2
10 suppliers
inquiry
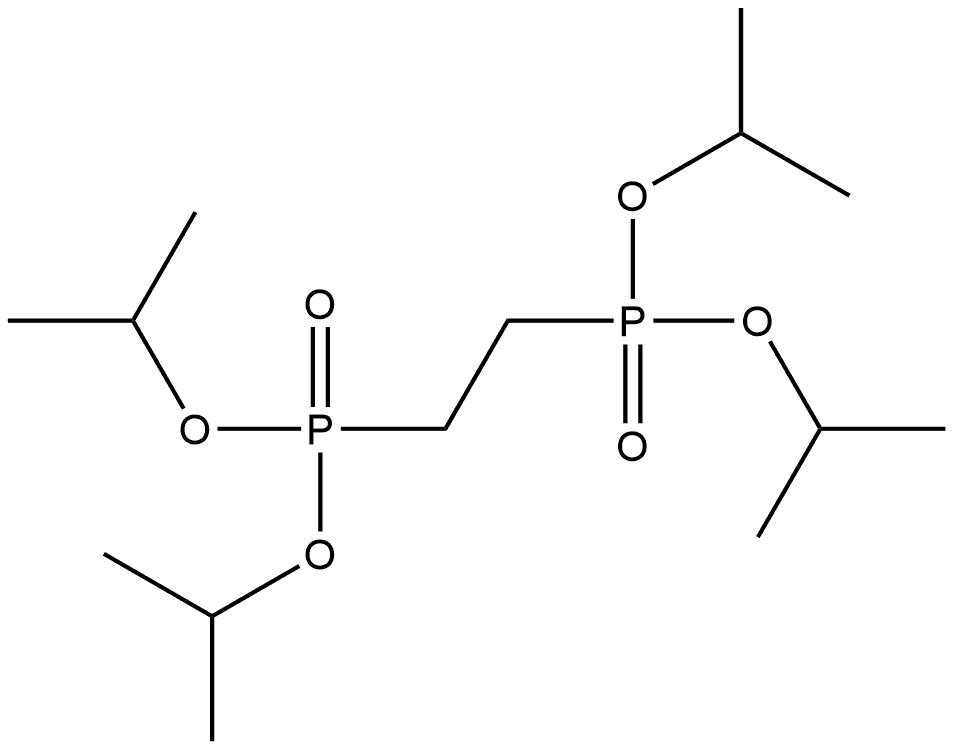
64630-16-6
1 suppliers
inquiry
