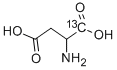
DL-ASPARTIC-1-13C ACID synthesis
- Product Name:DL-ASPARTIC-1-13C ACID
- CAS Number:137168-39-9
- Molecular formula:C4H7NO4
- Molecular Weight:134.11
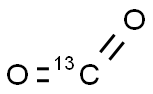
1111-72-4
51 suppliers
$350.00/1l
617-45-8
452 suppliers
$5.00/25g

137168-39-9
10 suppliers
$807.00/250mg
Yield:-
Reaction Conditions:
with caesium carbonate;4-methoxy-benzaldehyde in dimethyl sulfoxide at 70 - 90; under 760.051 Torr;
Steps:
General procedure for labelling α-amino acids with hydrophobic groups
General procedure: In an atmosphere-controlled glovebox, amino acid (0.20 mmol, 1.0 equiv.), 4-methoxybenzaldehyde (5.4 mg, 0.04 mmol, 0.20 equiv.), Cs2CO3 (26.1 mg, 0.08 mmol, 0.40 equiv.) and sodium trimethylsilylpropanesulfonate internal standard were sequentially added to an 8-dramvial charged with a stir bar, followed by the addition of anhydrous DMSO (2.0 ml). The vial was sealed with a PTFE-lined cap and removed from the glovebox. The reaction headspace was evacuated on a Schlenk line (~300 mTorr) using a 25-gauge needle. The vial headspace was then carefully refilled with 15 psi [13C]CO2 through the PTFE-lined cap witha 25-gauge needle, until the internal pressure reached ~1 atm (which requires 20-60 s, depending on the pressure of the 13CO2 tank). This provides ~8 equiv. (~1.6 mmol) of [13C]CO2, which would result in an equilibrium exchange incorporation of ~85%. The vial cap was then sealed with parafilm and electrical tape, and the reaction was stirred at 70 °C in an aluminium block. The vial was cooled to room temperature upon completion of the reaction (25-48 h). To determine the percentre covery of the amino acid, a small aliquot (~5 μl) of the reaction was placed in 0.70 ml D2O for calibrated 1H NMR analysis, using sodium trimethylsilylpropanesulfonate internal standard as the reference signal. After sampling, the reaction headspace was evacuated and refilled with N2. This cycle was repeated three times. Boc2O (91.9 μl, 0.40 mmol, 2.0equiv.) was then added via syringe and the reaction was stirred overnight at room temperature. After overnight stirring, a small aliquot of the reaction (~5 μl) was placed in 1.0 ml of 1:1 MeOH:H2O/0.1% HCO2H for LC-MS analysis to determine the crude 13C% incorporation of the amino acid. The reaction mixture was then diluted with H2O (10 ml) and acidified to pH 1-2 using 1 M HCl (2-3 ml). The aqueous layer was then extracted with EtOAc (5 × 10 ml). Next, the combined organics were washed withH2O (1 × 10 ml) and brine (1 × 10 ml). The organic layer was dried over anhydrous Na2SO4, filtered, concentrated in vacuo, and purified by silica gel chromatography. The 13C% incorporation was obtained through high-resolution mass spectrometry analysis of the products. Note that Boc protection of the products was done for convenience of isolation; this is not an essential step for carboxylate exchange. Note: use a 2-dramvial for 0.05-mmol-scale reactions; use a 4-dram vial for 0.10-mmol-scale reactions. A similar equilibrium incorporation (~85%) would be obtained under these conditions. Further details on procedures followed, including procedures, which do not require an atmosphere-controlled glovebox, can be found in Supplementary Section 2.
References:
Bsharat, Odey;Doyle, Michael G. J.;Munch, Maxime;Mair, Braeden A.;Cooze, Christopher J. C.;Derdau, Volker;Bauer, Armin;Kong, Duanyang;Rotstein, Benjamin H.;Lundgren, Rylan J. [Nature Chemistry,2022,vol. 14,# 12,p. 1367 - 1374]