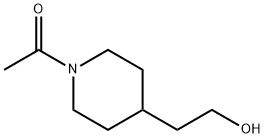
ETHANONE,1-[4-(2-HYDROXYETHYL)-1-PIPERIDINYL]- synthesis
- Product Name:ETHANONE,1-[4-(2-HYDROXYETHYL)-1-PIPERIDINYL]-
- CAS Number:15871-63-3
- Molecular formula:C9H17NO2
- Molecular Weight:171.24
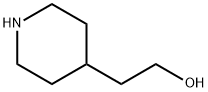
622-26-4
216 suppliers
$7.00/250mg

108-24-7
0 suppliers
$14.00/250ML
![ETHANONE,1-[4-(2-HYDROXYETHYL)-1-PIPERIDINYL]-](/CAS/GIF/15871-63-3.gif)
15871-63-3
10 suppliers
$450.00/500mg
Yield:15871-63-3 86%
Reaction Conditions:
in dichloromethane; for 2 h;Cooling with ice;
Steps:
3 In the synthetic route, the specific process of intermediate (9) (hereinafter referred to as compound 9) is:
Under an ice bath, compound 5 (abbreviation of (5) in the synthetic route) (2.00g, 15.5mmol) was dissolved in DC M solution (8mL), and acetic anhydride (1.10g, 17.0mmol) was slowly dropped into it, Stir under ice bath for 2 hours. After the reaction was completed, the reaction solution was washed with saturated sodium chloride (8 mL), dried over anhydrous sodium sulfate and concentrated to obtain compound 9 (colorless oily liquid, 2.28 g, 86%).
References:
CN113264937,2021,A Location in patent:Paragraph 0077-0079; 0083-0085

78056-59-4
2 suppliers
inquiry
![ETHANONE,1-[4-(2-HYDROXYETHYL)-1-PIPERIDINYL]-](/CAS/GIF/15871-63-3.gif)
15871-63-3
10 suppliers
$450.00/500mg
59184-90-6
130 suppliers
$11.00/250mg
![ETHANONE,1-[4-(2-HYDROXYETHYL)-1-PIPERIDINYL]-](/CAS/GIF/15871-63-3.gif)
15871-63-3
10 suppliers
$450.00/500mg