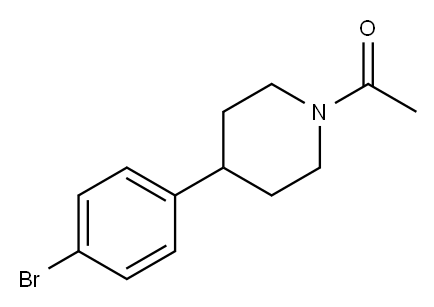
Ethanone, 1-[4-(4-bromophenyl)-1-piperidinyl]- synthesis
- Product Name:Ethanone, 1-[4-(4-bromophenyl)-1-piperidinyl]-
- CAS Number:176636-91-2
- Molecular formula:C13H16BrNO
- Molecular Weight:282.18
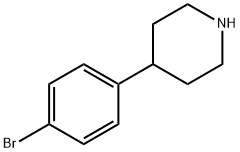
80980-89-8
137 suppliers
$40.00/500mg

108-24-7
0 suppliers
$14.00/250ML
![Ethanone, 1-[4-(4-bromophenyl)-1-piperidinyl]-](/CAS/20210111/GIF/176636-91-2.gif)
176636-91-2
3 suppliers
inquiry
Yield:176636-91-2 60%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 2 h;
Steps:
65.1 1-[4-(4-bromophenyl)piperidin-1-yl]ethanone
To a solution of 4-(4-bromophenyl)piperidine (400 mg, 1.70 mmol) in dichloromethane (20 mL) was added triethylamine (340 mg, 3.40 mmol) and acetic anhydride (500 mg, 5.10 mmol).
The mixture was stirred at room temperature for 2 hours.
The reaction mixture was concentrated under reduced pressure to give a crude residue.
The material was partitioned between water (20 mL) and ethyl acetate (20 mL).
The layers were separated and the aqueous layer was washed two additional times with ethyl acetate (30 mL).
The combined organics layers were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the title compound (260 mg, 60% yield) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.27 (d, 2H), 6.91 (d, 2H), 4.63 (d, 1H), 3.77 (d, 1H), 3.00 (t, 1H), 2.57-2.42 (t, 2H), 1.97 (s, 3H), 1.75-1.68 (m, 2H), 1.47-1.40 (m, 2H).
References:
US2013/267493,2013,A1 Location in patent:Paragraph 1003
769944-79-8
76 suppliers
$46.00/1/ G

75-36-5
589 suppliers
$17.92/100G
![Ethanone, 1-[4-(4-bromophenyl)-1-piperidinyl]-](/CAS/20210111/GIF/176636-91-2.gif)
176636-91-2
3 suppliers
inquiry