
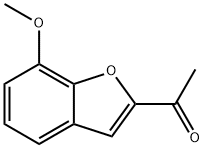
Ethanone, 1-(4-bromo-7-methoxy-2-benzofuranyl)- synthesis
- Product Name:Ethanone, 1-(4-bromo-7-methoxy-2-benzofuranyl)-
- CAS Number:192381-08-1
- Molecular formula:C11H9BrO3
- Molecular Weight:269.09
43071-52-9
112 suppliers
$17.00/1g

192381-08-1
7 suppliers
inquiry
Yield:192381-08-1 75%
Reaction Conditions:
with bromine;sodium acetate in acetic acid at 20; for 1 h;
Steps:
4.B Example 4; Intermediate B: Synthesis of 2-Acetyl-4-bromo-7-methoxybenzofuran
2-ACETYL-7-METHOXYBENZOFURAN (1.0 g, 5.3 mmol) was dissolved in glacial acetic acid (29 mL) followed by addition of sodium acetate (1.3 g, 15.8 MMOL). The reaction was treated dropwise with a solution of bromine (0.26 mL, 5. 26. mmol) in glacial acetic acid (10 mL) at room temperature followed by stirring for one hour. The solvent was removed under vacuum. The residue was dissolved in water and extracted three times with dichloromethane. The combined organic extracts were washed with 2% aqueous sodium bicarbonate, dried over sodium sulfate and concentrated under vacuum. The residue was purified on silica gel using a 50-100% dichloromethane/hexane gradient affording the product as a white solid. (1.00 g, 75%). 1H NMR (CDC13,300 MHz) 8 7. 44 (s, 1H), 7.32 (d, 1H), 6.80 (d, 1H), 4.0 (s, 3H), 2.62 (s, 3H).
References:
WO2004/94411,2004,A1 Location in patent:Page 109