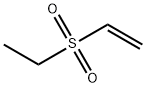
Ethene, (ethylsulfinyl)- synthesis
- Product Name:Ethene, (ethylsulfinyl)-
- CAS Number:32568-51-7
- Molecular formula:C4H8OS
- Molecular Weight:104.17
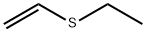
627-50-9
43 suppliers
$45.00/50mg

32568-51-7
0 suppliers
inquiry
Yield:32568-51-7 > 99 %Spectr.
Reaction Conditions:
with diphenyl diselenide;urea hydrogen peroxide adduct in dichloromethane at 20; for 24 h;
Steps:
Sulfoxide from ethyl vinyl sulfide
General procedure: UHP (2 mmol) was dissolved in CH2Cl2 (2 mL), and the solutionwas stirred at r.t. A solution of Ph2Se2 (1 mol%) and sulfide (2mmol) in CH2Cl2 (2 mL) was added to the UHP solution. Themixture was stirred at r.t. for 24 h or until complete conversionto sulfoxide was observed by TLC. Extraction was carried outwith CH2Cl2 (3 × 5 mL), after the addition of H2O (5 mL), and thecombined organic solutions were washed with brine (50 mL),dried (MgSO4), filtered, and the solvents removed underreduced pressure. Sulfoxide products were purified where necessaryby column chromatography. Yellow oil; νmax (cm-1) 2950, 2891, 1657, 1438, 1019, 743; 1H NMR (400 MHz, CDCl3): δH 1.29 (3H, t, J 7.2 Hz), 2.65 (1 H, dq, J 7.4 Hz), 2.85 (1 H, dq, J 7.4 Hz), 5.99 (1 H, d, J 10.0 Hz), 6.10 (1H, d, J 16.8 Hz), 6.58 (1 H, dd, J 10.0 & 16.8 Hz) ppm; 13C NMR (100 MHz, CDCl3): δC 5.6, 46.5,48.6, 122.6, 139.8
References:
Bulman Page, Philip C.;Buckley, Benjamin R.;Elliott, Claire;Chan, Yohan;Dreyfus, Nicolas;Marken, Frank [Synlett,2016,vol. 27,# 1,art. no. ST-2015-D0481-L,p. 80 - 82] Location in patent:supporting information
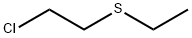
693-07-2
131 suppliers
$35.00/1g

32568-51-7
0 suppliers
inquiry

27998-62-5
11 suppliers
inquiry
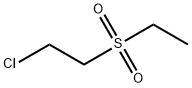
25027-40-1
35 suppliers
$45.00/50mg

27998-62-5
11 suppliers
inquiry

32568-51-7
0 suppliers
inquiry