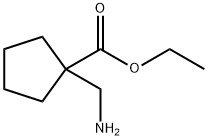
ETHYL 1-(AMINOMETHYL)CYCLOPENTANECARBOXYLATE synthesis
- Product Name:ETHYL 1-(AMINOMETHYL)CYCLOPENTANECARBOXYLATE
- CAS Number:99065-34-6
- Molecular formula:C9H17NO2
- Molecular Weight:171.24
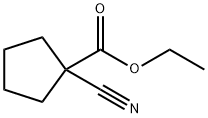
28247-14-5
63 suppliers
inquiry

99065-34-6
23 suppliers
inquiry
Yield:99065-34-6 71%
Reaction Conditions:
with hydrogen in ethanol;lithium hydroxide monohydrate at 0.2 - 0.3; for 48 h;
Steps:
74.1 Step 1. Ethyl 1-(amino ylate
To a solution of ethyl 1-cyanocy 1-carboxylate (10.0 g, 59.8 mmol) in ethanol (30 mL) was added Raney-Nickel (1.43 g, 24.3 mmol; slurry in water; Aldrich, 221678). The mixture was stirred under an atmosphere of H2at room temperature for 48 h. The mixture was filtered through Celite and concentrated. The crude residue was dissolved in DCM, dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (0-20% MeOH/DCM) to afford the title compound (7.30 g, 42.6 mmol, 71.0% yield) as a colorless oil.1H NMR (300 MHz, CDCl3) δ 4.13 (q, J = 7.1 Hz, 2H), 2.80 (s, 2H), 2.09 - 1.97 (m, 2H), 1.86 (s, 2H), 1.74 - 1.43 (m, 6H), 1.24 (t, J = 7.1 Hz, 3H). Rf= 0.2 (10% MeOH/DCM, ninhydrin stain).
References:
WO2022/133215,2022,A1 Location in patent:Paragraph 00395; 00558-00559
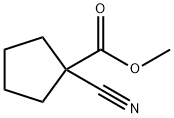
40862-12-2
52 suppliers
inquiry

99065-34-6
23 suppliers
inquiry
![2-[2-[(E)-2-[4-(dimethylamino)phenyl]ethenyl]-6-methylpyran-4-ylidene]propanedinitrile](/CAS/20180601/GIF/96042-30-7.gif)