
Ethyl 1-chloroisoquinoline-3-carboxylate synthesis
- Product Name:Ethyl 1-chloroisoquinoline-3-carboxylate
- CAS Number:1256353-08-8
- Molecular formula:C12H10ClNO2
- Molecular Weight:235.67

94726-24-6
7 suppliers
inquiry

1256353-08-8
10 suppliers
inquiry
Yield:1256353-08-8 66%
Reaction Conditions:
Stage #1: ethyl 1,2-dihydro-1-oxo-isoquinoline-3-carboxylatewith trichlorophosphate for 2 h;Reflux;
Stage #2: with sodium hydrogencarbonate at 20; pH=7;Cooling with ice;
Steps:
I.C
Step C: A stirred mixture of ethyl l-oxo-l,2-dihydroisoquinoline-3- carboxylate (1.8 g, 0.23 mmol) in phosphorus oxychloride (15 mL) was heated under reflux for 2 h. After cooling to rt, the mixture was poured into ice water and adjusted to pH 7 with aq sodium hydrogen carbonate. The mixture was extracted with EtOAc and the combined organic layers were dried over Na2S04, filtered, and concentrated under reduced pressure to afford ethyl l-chloroisoquinoline-3-carboxylate as a tan solid (1.3 g, 66%). 1H NMR (400 MHz, CDC13) δ 8.54 (s, 1H), 8.45 (d, J= 7.6 Hz, 1H), 8.03 (d, J= 7.2 Hz, 1H), 7.85-7.87 (m, 2H), 4.55 (q, J= 7.2 Hz, 2H), 1.50 (t, J = 7.2 Hz, 3H); LC-MS (ESI) m/z 236 (M+H)+.
References:
WO2012/30944,2012,A2 Location in patent:Page/Page column 81
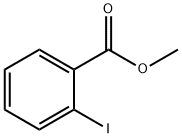
610-97-9
427 suppliers
$9.00/1g

1256353-08-8
10 suppliers
inquiry
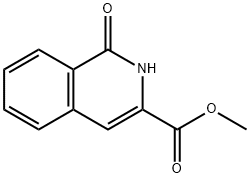
69454-42-8
30 suppliers
inquiry

1256353-08-8
10 suppliers
inquiry