
ethyl 1-ethyl-3-methyl-1H-pyrazole-4-carboxylate synthesis
- Product Name:ethyl 1-ethyl-3-methyl-1H-pyrazole-4-carboxylate
- CAS Number:1451192-48-5
- Molecular formula:C9H14N2O2
- Molecular Weight:182.22
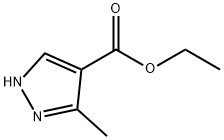
85290-78-4
120 suppliers
$7.00/250mg

75-03-6
365 suppliers
$11.00/5g

1451192-48-5
2 suppliers
inquiry

1343032-27-8
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: ethyl 3-methylpyrazole-4-carboxylatewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.0833333 h;
Stage #2: ethyl iodide in N,N-dimethyl-formamide;mineral oil at 20;Overall yield = 47 percent; Overall yield = 840 mg;
Steps:
2A 2A Ethyl 1-ethyl-3-methyl-1H-pyrazole-4-carboxylate
NaH (60% in mineral oil; 0.300 g, 7.50 mmol) was added portionwise to a solution of ethyl 3-methyl-1H-pyrazole-4-carboxylate (1.00 g, 6.49 mmol) in DMF (10 mL) at 0 °C The reaction mixture was stirred for 5 min, iodoethane (0.60 mL, 7.42 mmol) added and the reaction mixture stirred at rt overnight. The reaction mixture was quenched with water (50 mL), diluted with MTBE (50 mL), separated and the aqueous phase extracted with MTBE (2 × 20 mL). The combined organics were washed with 1 : 1 water/brine (2 × 50 mL), dried (MgSO4), and the solvent removed under reduced pressure. Purification by flash chromatography (0-30% EtO Ac/isohexanes) afforded a pale yellow oil (840 mg, 47%). Material was ~1:0.8 mixture of the desired regioisomer with ethyl 1-ethyl-5- methyl-1H-pyrazole-4-carboxylate as the minor product. Used without further purification. Ratio ascertained by1H NMR based upon d pyrazole CH signal:1H NMR (500 MHz, CDCl3) δ 7.83 (s, 1H), 4.27 (q, J= 7.1 Hz, 2H), 4.11 (q, J= 7.3 Hz, 2H), 2.46 (s, 3H), 1.48 (t, J= 7.3 Hz, 3H), 1.34 (t, J= 7.1 Hz, 3H). LCMS (method A) m/z 183.1 [M+H]+at 1.00 min. Minor product: ethyl 1-ethyl-5-methyl-1H-pyrazole-4- carboxylate.1H NMR (500 MHz, CDCl3) δ 7.84 (s, 1H), 4.28 (q, 7= 7.1 Hz, 2H), 4.11 (q, J = 7.3 Hz, 2H), 2.54 (s, 3H), 1.41 (t, J = 7.3 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). LCMS (method A) m/z 183.1 [M+H]+at 1.00 min.
References:
WO2021/79121,2021,A1 Location in patent:Page/Page column 26
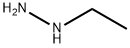
624-80-6
36 suppliers
inquiry
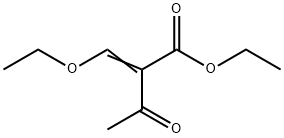
3788-94-1
169 suppliers
$11.00/1g

1451192-48-5
2 suppliers
inquiry

1343032-27-8
0 suppliers
inquiry
