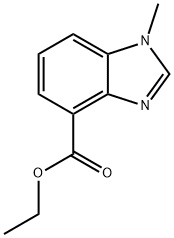
Ethyl 1-Methyl-4-benzimidazolecarboxylate synthesis
- Product Name:Ethyl 1-Methyl-4-benzimidazolecarboxylate
- CAS Number:856840-76-1
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23

74-88-4
340 suppliers
$15.00/10g

856840-76-1
20 suppliers
inquiry
Yield:856840-76-1 76%
Reaction Conditions:
Stage #1: ethyl 1H-1,3-benzodiazole-4-carboxylatewith sodium hydride in DMF (N,N-dimethyl-formamide) at 0 - 5; for 1 h;
Stage #2: methyl iodide in DMF (N,N-dimethyl-formamide) at 0 - 20; for 2 h;
Steps:
19; S
NaH (0.054 mol) was added portion wise at 5°C under N2 flow to a mixture of lH-benzoimidazole-4-carboxylic acid ethyl ester (0. 045 mol) in DMF (50 ml). The mixture was stirred at 0°C under N2 flow for 1 hour. CH3I (0.045 mol) was added drop wise at 0°C under N2 flow. The mixture was stirred at room temperature under N2 flow for 2 hours, hydrolyzed with ice water and extracted with CH2C12. The organic layer was separated, washed several times with H2O, dried (over MgSO4), filtered and the solvent was evaporated. The residue (10.5 g) was purified by column chromatography over silica gel (eluent: CH2CI2/CH30H 97/3 ; 20-45 um). Two pure fractions were collected and their solvents were evaporated. Yield: 7 g of intermediate s-2 (76%, melting point : 86°C).
References:
WO2005/58869,2005,A1 Location in patent:Page/Page column 54-55
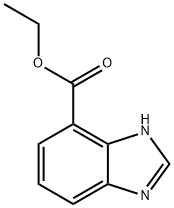
167487-83-4
58 suppliers
$98.29/250mg

74-88-4
340 suppliers
$15.00/10g

856840-76-1
20 suppliers
inquiry