
ETHYL 1-METHYL-5-NITROIMIDAZOLE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 1-METHYL-5-NITROIMIDAZOLE-2-CARBOXYLATE
- CAS Number:1564-49-4
- Molecular formula:C7H9N3O4
- Molecular Weight:199.16
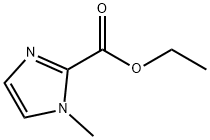
30148-21-1

1564-49-4
Step 6a. ethyl 1-methyl-1H-imidazole-2-carboxylate (2.0 g, 13 mmol) was dissolved in 8 mL of concentrated H2SO4, followed by the slow addition of 8 mL of concentrated HNO3. The reaction mixture was stirred at 70 °C for 3 h. The reaction mixture was then extracted with dichloromethane (DCM). After completion of the reaction, the solution was diluted with H2O and the pH was adjusted to 7-8 with aqueous Na2CO3. The reaction mixture was extracted with dichloromethane (DCM) and the organic layers were combined and concentrated. Purification by silica gel column chromatography (eluent: ethyl acetate/petroleum ether) afforded the target compound 1-methyl-5-nitro-1H-imidazole-2-carboxylic acid ethyl ester as a yellow solid (0.9 g, 34.7% yield).ESI-MS m/z = 200.20 [M + H]+.

30148-21-1
155 suppliers
$27.00/1g

1564-49-4
43 suppliers
inquiry
Yield: 34.7%
Reaction Conditions:
with sulfuric acid;nitric acid at 70; for 3 h;
Steps:
6.6a Step 6a.
Step 6a. A solution of ethyl 1 -methyl- lH-imidazole-2-carboxy late (2.0 g, 13 mmol) in 8 ml cone. H2SO4 and 8 ml cone. HNO3 was stirred for 3 hours at 70°C. The solution was diluted with H2O, then adjusted to pH 7-8 with aq Na2C03 solution and extracted with DCM. The organic layer was concentrated and chromatographed (silica, ethyl acetate/petroleum ether) to give the desired compound as yellow solid (0.9g, 34.7%). ESI- MS m/z = 200.20 [M+H]+
References:
ENANTA PHARMACEUTICALS, INC.;QIU, Yao-ling;LI, Wei;CAO, Hui;JIN, Meizhong;GAO, Xuri;PENG, Xiaowen;KASS, Jorden;OR, Yat, Sun WO2016/183266, 2016, A1 Location in patent:Page/Page column 50

30148-21-1
155 suppliers
$27.00/1g
109012-23-9
84 suppliers
$12.00/250mg
109012-22-8
0 suppliers
inquiry

1564-49-4
43 suppliers
inquiry