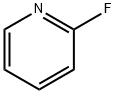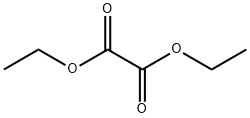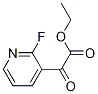
ethyl 2-(2-fluoropyridin-3-yl)-2-oxoacetate synthesis
- Product Name:ethyl 2-(2-fluoropyridin-3-yl)-2-oxoacetate
- CAS Number:849069-30-3
- Molecular formula:C9H8FNO3
- Molecular Weight:197.16
Yield:849069-30-3 34.9%
Reaction Conditions:
with lithium dipropan-2-ylazanide in tetrahydrofuran at -78 - 20; for 2 h;Inert atmosphere;
Steps:
Ethyl 2-(2-fluoropyridin-3-yl)-2-oxoacetate (QY-9-10):
2-Fluoropyridine (5.0 g, 51.5 mmol) was added to a 250 ml round-bottomed flask, and 120 ml of tetrahydrofuran was added to dissolve and stir evenly. After nitrogen replacement protection, the reaction system was transferred to -78°C, and lithium diisopropylamide was slowly added dropwise. (30.4 ml, 61.8 mmol) into the reaction solution, after the dropwise addition, diethyl oxalate (9.0 g, 61.8 mmol) was added, gradually returned to room temperature, and then continued to stir for 2 h, and the reaction was monitored in real time by LC-MS until the reactant was completely consumed, Add saturated ammonium chloride to quench the reaction, transfer the reaction system to a separatory funnel, extract with ethyl acetate (20ml*3), combine the organic phases, wash with saturated sodium chloride (15ml*2), dry the organic phase with anhydrous sodium sulfate , filtration and rotary evaporation to remove ethyl acetate, and separation and purification by silica gel column chromatography (petroleum ether: ethyl acetate = 0-40%) to obtain 3.54 g of a bright yellow oily liquid with a yield of 34.9%.
References:
WO2022/57787,2022,A1 Location in patent:Page/Page column 37