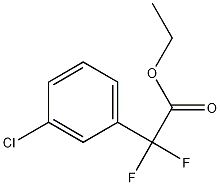
Ethyl 2-(3-chlorophenyl)-2,2-difluoroacetate synthesis
- Product Name:Ethyl 2-(3-chlorophenyl)-2,2-difluoroacetate
- CAS Number:135334-14-4
- Molecular formula:C10H9ClF2O2
- Molecular Weight:234.63
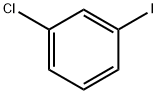
625-99-0
227 suppliers
$5.00/1g

135334-14-4
28 suppliers
$120.00/250mg
Yield: 97%
Reaction Conditions:
Stage #1:ethyl 3-bromo-3,3-difluoropropanoate with copper bronze in dimethyl sulfoxide at 20; for 1 h;
Stage #2:3-iodochlorobenzene in dimethyl sulfoxide at 20; for 72 h;
Steps:
2.a EXAMPLE 2 N-[2-(Amidinoaminooxy)ethyl]-2-{3-[2-(3-chlorophenyl)-2,2-difluoroethylamino]-6-cyano-2-fluorophenyl}acetamide a. (3-Chlorophenyl)difluoroacetic acid ethyl ester
To a mixture of copper bronze (2.93 g, 46.1 mmol) in DMSO (5.0 mL) was added ethyl bromodifluoroacetate (3.2 mL, 25 mmol) under argon at room temperature (see Sato, K. et al; Chem. Pharm. Bull. 47:1013-1016, 1999). After stirring for 1 h, 1-chloro-3-iodobenzene (2.57 mL, 20.8 mmol) was added under argon at room temperature. After shaking for 3 days, the mixture was diluted with saturated NH4Cl (50 mL) and extracted with ether (25 mL). The biphasic mixture was filtered through a pad of Celite, and the filter cake was washed with ether (25 mL). The organic layer was separated from the filtrate, and the aqueous layer was extracted with ether (25 mL). The combined organic layers were washed with saturated NH4Cl (2×15 mL) and brine (15 mL), dried (Na2SO4), and concentrated to afford the title compound (4.77 g, 97%) as a clear yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.63-7.59 (m, 1H), 7.53-7.45 (m, 2H), 7.43-7.36 (m, 1H), 4.31 (q, J=7.2 Hz, 2H), 1.32 (t, J=7.2 Hz, 3H).
References:
Kreutter, Kevin D.;Lee, Lily;Lu, Tianbao;Mohan, Venkatraman;Patel, Sharmila;Huang, Hui;Xu, Guozhang;Fitzgerald, Mark US2004/254166, 2004, A1 Location in patent:Page 20-21

625-99-0
227 suppliers
$5.00/1g
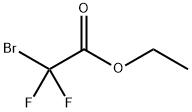
667-27-6
418 suppliers
$10.00/10 g

135334-14-4
28 suppliers
$120.00/250mg

667-27-6
418 suppliers
$10.00/10 g
86364-03-6
25 suppliers
inquiry

135334-14-4
28 suppliers
$120.00/250mg