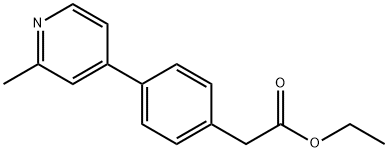
ethyl 2-(4-(2-methylpyridin-4-yl)phenyl)acetate synthesis
- Product Name:ethyl 2-(4-(2-methylpyridin-4-yl)phenyl)acetate
- CAS Number:1243245-68-2
- Molecular formula:C16H17NO2
- Molecular Weight:255.31
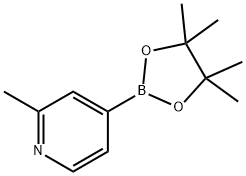
660867-80-1
166 suppliers
$11.00/250mg
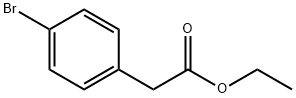
14062-25-0
255 suppliers
$6.00/5g

1243245-68-2
8 suppliers
inquiry
Yield:1243245-68-2 500 mg
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);caesium carbonate in tetrahydrofuran at 75; for 12 h;Inert atmosphere;
Steps:
25 Ethyl 2-(4-(2-methylpyridin-4-yl)phenyl)acetate (54)
To a suspension of 4-bromo-2-methylpyridine (500 mg, 2.90 mmol), bis(pinacolato)diboron (795 mg, 3.13 mmol) and Pd(dppf)Cl2 (42 mg, 0.058 mmol) in THF (10 mL) was added KOAc (568 mg, 5.80 mmol).
The reaction mixture was stirred at 80 °C under N2 overnight.
After cooling to room temperature, the mixture was diluted with dichloromethane (200 mL) and then filtered.
The filtration was concentrated to give the desired product (1.27 g, crude) as a black oil, which was used directly in the next step without further purification.
To a suspension of the above crude oil (1.27 g), ethyl 2-(4-bromophenyl)acetate (640 mg, 2.63 mmol) and Pd(PPh3)4 (243 mg, 0.21 mmol) in THF (10 mL) was added Cs2CO3 (1.7 g, 5.26 mmol).
The mixture was stirred at 75 °C under N2 for 12 h.
After cooling to room temperature, the mixture was evaporated and the residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 5/1) to give the desired product (500 mg, 75%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 5.2 Hz, 1H), 7.57 (d, J = 8.0 Hz, 2H), 7.38 (d, J = 8.0 Hz, 2H), 7.34 (s, 1H), 7.29 (d, J = 5.2 Hz, 1H), 4.16 (q, J = 7.2 Hz, 2H), 3.66 (s, 2H), 2.60 (s, 3H), 1.26 (t, J = 7.2 Hz, 3H).
References:
Xu, Zhixiang;Li, Jiajun;Wu, Yiyuan;Sun, Zhijian;Luo, Lusong;Hu, Zhilin;He, Sudan;Zheng, Jiyue;Zhang, Hongjian;Zhang, Xiaohu [European Journal of Medicinal Chemistry,2016,vol. 108,p. 154 - 165]

660867-80-1
166 suppliers
$11.00/250mg
15250-46-1
65 suppliers
$11.00/250mg

1243245-68-2
8 suppliers
inquiry
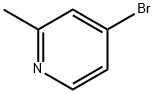
22282-99-1
405 suppliers
$6.00/5g

1243245-68-2
8 suppliers
inquiry