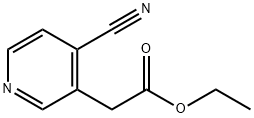
ethyl 2-(4-cyanopyridin-3-yl)acetate synthesis
- Product Name:ethyl 2-(4-cyanopyridin-3-yl)acetate
- CAS Number:3423-46-9
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2
Yield:3423-46-9 47.63%
Reaction Conditions:
at 45; for 6 h;
Steps:
1.264.2 Step 2
Into a 500 mL round-bottom flask, was placed 3-(2-ethoxy-2-oxoethyl)pyridin- 1-ium-1-olate (20.00 g, 110.38 mmol, 1.00 equiv), ethyl iodide (51.65 g, 331.16 mmol, 3.00 equiv). The resulting solution was stirred for 6 h at 45 °C. The resulting solution was added CH3CN (350.00 mL), K2CO3 (45.77 g, 331.14 mmol, 3.00 equiv), TMSCN (32.85 g, 331.14 mmol, 3.00 equiv). The resulting solution was stirred for overnight at 50 °C. The reaction mixture was cooled to room temperature. The solids were filtered out and the filtrate was concentrated. The residue was applied onto a silica gel column with THF/PE (10%). This resulted in 10 g (47.63%) of ethyl 2-(4-cyanopyridin-3-yl)acetate as yellow oil. LCMS (ES) [M+1]+ m/z: 191.
References:
WO2021/222483,2021,A1 Location in patent:Paragraph 1325; 1327
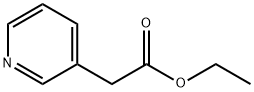
39931-77-6
188 suppliers
$10.00/1g

3423-46-9
11 suppliers
inquiry
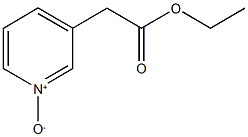
3423-47-0
3 suppliers
inquiry

3423-46-9
11 suppliers
inquiry
