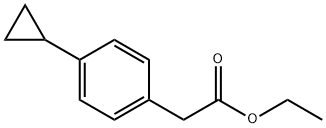
ethyl 2-(4-cyclopropylphenyl)acetate synthesis
- Product Name:ethyl 2-(4-cyclopropylphenyl)acetate
- CAS Number:40641-92-7
- Molecular formula:C13H16O2
- Molecular Weight:204.26
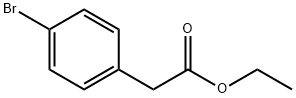
14062-25-0
254 suppliers
$6.00/5g
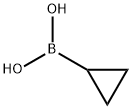
411235-57-9
427 suppliers
$6.00/1g

40641-92-7
16 suppliers
$524.00/1g
Yield: 97%
Reaction Conditions:
with tripotassium phosphate "n" hydrate;palladium diacetate;tricyclohexylphosphine in water;toluene at 100; for 16 h;Inert atmosphere;
Steps:
33-1 Ethyl 2-(4-cyclopropylphenyl)acetate
Ethyl 4-bromophenylacetate (6.0 g, 24.7 mmol), cyclopropylboronic acid (2.76 g, 32.1 mmol), palladium acetate (276 mg, 1.23 mmol), tricyclohexylphosphine (0.6 M toluene solution, 4.2 mL, 2.46 mmol), and potassium phosphate monohydrate (19.9 g, 86.4 mmol) were suspended in toluene (60.0 mL) and water (3.0 mL), and the suspension was stirred for 16 hours at 100°C under a nitrogen gas stream. The reaction liquid that had been left to cool to room temperature was filtered through a Celite pad, and then the solvent of the filtrate was distilled off under reduced pressure. A residue thus obtained was diluted with ethyl acetate, an organic layer was washed with an aqueous solution of sodium hydrogen carbonate, water, and saturated brine and then was dried over anhydrous sodium sulfate, insoluble materials were filtered, and then the solvent was distilled off under reduced pressure. A residue thus obtained was purified by silica gel column chromatography (heptane : ethyl acetate) (concentration gradient: 0 to 30%), and the title compound (yellow oily material, 4.90 g, 97%) was obtained. 1H NMR (CDCl3, 400MHz) : δ=0.6-0.7 (m, 2H), 0.9-1.0 (m, 2H), 1.24 (t, 3H, J=8Hz), 1.8-1.9 (m, 1H), 3.52 (s, 2H), 4.12 (q, 2H, J=8Hz), 7.01 (d, 2H, J=8Hz), 7.15 (d, 2H, J=8Hz).
References:
Nippon Chemiphar Co., Ltd.;Kinki University EP3825303, 2021, A1 Location in patent:Paragraph 0237-0238

14062-25-0
254 suppliers
$6.00/5g
23719-80-4
177 suppliers
$85.20/25ml

40641-92-7
16 suppliers
$524.00/1g