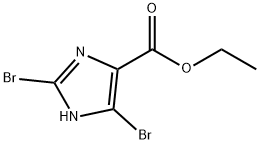
ETHYL 2,4-DIBROMO-1H-IMIDAZOLE-5-CARBOXYLATE synthesis
- Product Name:ETHYL 2,4-DIBROMO-1H-IMIDAZOLE-5-CARBOXYLATE
- CAS Number:74478-96-9
- Molecular formula:C6H6Br2N2O2
- Molecular Weight:297.93
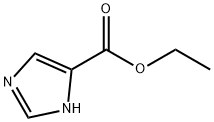
23785-21-9
0 suppliers
$6.00/250mg

74478-96-9
34 suppliers
inquiry
Yield:74478-96-9 74%
Reaction Conditions:
with N-Bromosuccinimide in ethanol at 20; for 4 h;
Steps:
10.1 ethyl 2,4-dibromo-1H-imidazole-5-carboxylate
Step 1
ethyl 2,4-dibromo-1H-imidazole-5-carboxylate
To a solution of ethyl 4H-imidazole-4-carboxylate (6.4 g, 50 mmol, 1.00 equiv) in ethanol (50 mL) was added NBS (17.8 g, 100 mmol, 2.00 equiv) in portions.
The reaction was stirred at room temperature for 4 h and then concentrated under vacuum.
The residue was purified on a silica gel column eluted with 0-10% of ethyl acetate in petroleum ether to give 3.6 g (74%) of ethyl 2,4-dibromo-1H-imidazole-5-carboxylate as a white solid. 1H-NMR (300 MHz, CDCl3): δ 4.45-4.38 (m, 2H), 1.41 (t, J=9.6 Hz, 3H) ppm.
References:
US2014/315961,2014,A1 Location in patent:Paragraph 0407-0408

23785-21-9
0 suppliers
$6.00/250mg

74478-96-9
34 suppliers
inquiry