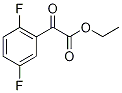
Ethyl 2,5-difluorobenzoylformate synthesis
- Product Name:Ethyl 2,5-difluorobenzoylformate
- CAS Number:1049131-01-2
- Molecular formula:C10H8F2O3
- Molecular Weight:214.17
Yield:1049131-01-2 62%
Reaction Conditions:
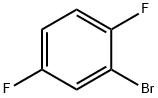
Stage #1: 2-bromo-1,4-difluorobenzenewith magnesium in tetrahydrofuran;
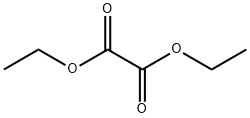
Stage #2: oxalic acid diethyl ester in tetrahydrofuran at -40 - 0; for 1 h;
Steps:
71
Reference Example-71
THF (25 mL) was added to metal magnesium (2.55 g, 105 mmol), and 2-bromo-1,4-difluoro benzene (19.70 g, 100 mmol) was slowly added thereto, whereby a Grignard reagent was prepared.
The previously prepared Grignard reagent was added dropwise to the separately prepared solution of diethyl oxalate (15.34 g, 105 mmol) in THF (10 mL) at -40° C. or lower.
After the dropping was completed, the reaction temperature was raised to 0° C., followed stirring for 1 hour.
A saturated ammonium chloride aqueous solution and water (200 mL) were added to the reaction solution, and the resultant product was extracted with ethyl acetate (200 mL*2).
After the organic layer was dried over magnesium sulfate and filtered, the solvent was distilled off under reduced pressure, and the resultant product was distilled under reduced pressure, whereby ethyl 2-(2,5-difluorophenyl)-2-oxoacetate (14.82 g, yield: 62%) was obtained as a colorless liquid. 1H-NMR (400 MHz, CDCl3): δ1.40 (t, J=7.0 Hz, 3H), 4.44 (q, J=7.0 Hz, 2H), 7.16 (ddd, J=9.3, 9.3 and 4.0 Hz, 1H), 7.34 (dddd, J=9.3, 9.2, 7.3 and 3.3 Hz, 1H), 7.60 (ddd, J=8.2, 5.3 and 3.3 Hz, 1H).
19F-NMR (376 MHz, CDCl3): δ-116.9 (d, J=7.8 Hz, 1F), -116.3 (d, J=7.8 Hz, 1F).
References:
US2016/24110,2016,A1 Location in patent:Paragraph 1225; 1226