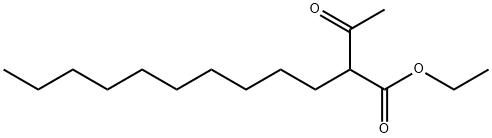
Ethyl 2-acetyldodecanoate synthesis
- Product Name:Ethyl 2-acetyldodecanoate
- CAS Number:40778-32-3
- Molecular formula:C16H30O3
- Molecular Weight:270.41
Yield:40778-32-3 98%
Reaction Conditions:
Stage #1: ethyl acetoacetatewith sodium hydride in tetrahydrofuran;mineral oil at 0 - 23; for 0.166667 h;Inert atmosphere;
Stage #2: Iododecane in tetrahydrofuran;mineral oil at 66;Inert atmosphere;
Steps:
ethyl 2-diazododecanoate (16): Alkylation
To a flame-dried round-bottom flask equipped with a magnetic stir bar were added sodium hydride (60% suspension in mineral oil, 600 mg, 15.0 mmol, 1.50 equiv) and dry THF (15 mL). The contents were stirred under nitrogen and cooled to 0 °C using an ice water bath. Ethyl acetoacetate (S17, 1.90 mL, 15.0 mmol, 1.50 equiv) was added dropwise by syringe, and the suspension was allowed to warm to 23 °C and stir for 10 minutes. n-Decyl iodide (2.13 mL, 10.0 mmol, 1.00 equiv) was added dropwise by syringe, and the suspension heated to reflux overnight. Upon completion (as determined by TLC analysis), the reaction mixture was quenched with saturated aqueous ammonium chloride (100 mL) and extracted with dichloromethane (3 x 100 mL). The combined organic layers were washed with brine (1 x 100 mL), dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The crude residue was purified by silica gel column chromatography (5% ethyl acetate in hexanes) to provide the alkylation product as a colorless oil (2.65 g, 98% yield).
References:
O'Connor, Nicholas R.;Bolgar, Peter;Stoltz, Brian M. [Tetrahedron Letters,2016,vol. 57,# 8,p. 849 - 851] Location in patent:supporting information