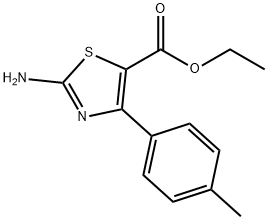
ETHYL 2-AMINO-4-P-TOLYLTHIAZOLE-5-CARBOXYLATE synthesis
- Product Name:ETHYL 2-AMINO-4-P-TOLYLTHIAZOLE-5-CARBOXYLATE
- CAS Number:68301-49-5
- Molecular formula:C13H14N2O2S
- Molecular Weight:262.33
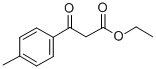
27835-00-3
125 suppliers
$13.00/250mg
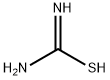
17356-08-0
2 suppliers
inquiry

68301-49-5
12 suppliers
$28.60/250mg
Yield:68301-49-5 41%
Reaction Conditions:
with ammonium iodide;lithium perchlorate;Alanine in water;dimethyl sulfoxide at 30;Electrolysis;
Steps:
General procedure for the one-pot electrochemically synthesis of 2-aminothiazoles from active methylene ketones and thioureas
General procedure: A 50 mL undivided cell was equipped with a graphite plate cathode and a graphiteplate anode (each about 2 × 2 cm2) which were connected to a DC regulated powersupply. To the cell was added active methylene ketone 1 (2 mmol), thiourea 2 (1 mmol),NH4I (0.1 mmol), DL-alanine (1 mmol) and LiClO4 (0.5 mmol) dissolved in a mixedsolvent of DMSO (1 mL) and H2O (14 mL). The mixture was electrolyzed underconstant current conditions at 5 mA/cm2 at 30 °C while stirring. The electrolysis wasterminated when 6 F/mol of charge had been consumed. After the electrolysis, thereaction mixture was washed with a saturated aqueous Na2S2O3 and the product wasthen extracted with DCM (3 × 10 mL), dried over MgSO4, and concentrated in vacuum.The residue was purified by column chromatography on silica gel using a mixture ofpetroleum ether/EtOAc as eluent.
References:
Yang, Shang-Feng;Li, Pei;Fang, Zi-Lin;Liang, Sen;Tian, Hong-Yu;Sun, Bao-Guo;Xu, Kun;Zeng, Cheng-Chu [Beilstein Journal of Organic Chemistry,2022,vol. 18,p. 1249 - 1255] Location in patent:supporting information