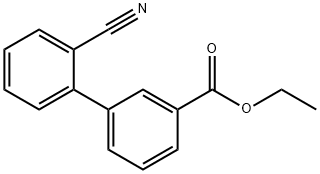
ETHYL 2'-CYANOBIPHENYL-3-CARBOXYLATE synthesis
- Product Name:ETHYL 2'-CYANOBIPHENYL-3-CARBOXYLATE
- CAS Number:131379-35-6
- Molecular formula:C16H13NO2
- Molecular Weight:251.28
Yield:131379-35-6 92%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in N,N-dimethyl-formamide at 100;Inert atmosphere;Suzuki Coupling;
Steps:
4.5.4. General procedure for Suzuki coupling reaction, compounds21a-e
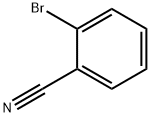
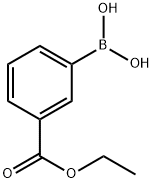
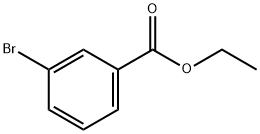
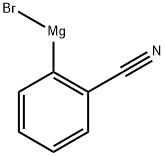
General procedure: To a three-neck round-bottom flask under argon atmospherewas added Pd(PPh3)4 (0.14 mmol), DMF (9.5 mL) and commerciallyavailable 2-, 3- or 4-bromobenzonitrile (1.41 mmol). K2CO3(4.24 mmol) and the corresponding 2-, 3- or 4-ethoxycarbonylbenzeneboronic acid (2.40 mmol) were successivelyadded and the reaction mixture was stirred under argon at100 C until TLC revealed that the starting material was consumed.The mixture was cooled to room temperature, diluted with waterand product was extracted with EtOAc. Organic layers were driedover MgSO4, concentrated in vacuum and the residue was purifiedby silica gel column chromatography (petroleum ether/EtOAc,0-60%) to provide the desired compounds.
References:
Guillon, Rémi;Rahimova, Rahila;Preeti;Egron, David;Rouanet, Sonia;Dumontet, Charles;Aghajari, Nushin;Jordheim, Lars Petter;Chaloin, Laurent;Peyrottes, Suzanne [European Journal of Medicinal Chemistry,2019,vol. 168,p. 28 - 44]