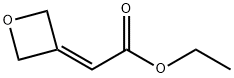
ethyl 2-(oxetan-3-ylidene)acetate synthesis
- Product Name:ethyl 2-(oxetan-3-ylidene)acetate
- CAS Number:922500-91-2
- Molecular formula:C7H10O3
- Molecular Weight:142.15
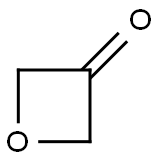
6704-31-0
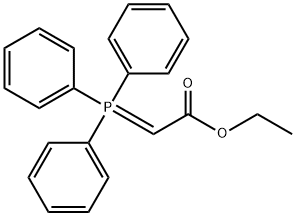
1099-45-2

922500-91-2
Oxetan-3-one (500 mg, 6.938 mmol) was dissolved in dichloromethane (15.00 mL) at 0 °C, followed by the addition of ethoxycarbonylmethylene triphenylphosphine (2.659 g, 7.632 mmol). The reaction system was slowly warmed to room temperature and stirred continuously for 15 minutes. Upon completion of the reaction, the reaction mixture was filtered through a pad of silica gel (using 30% ethyl acetate/petroleum ether as eluent) and the filtrate was concentrated under reduced pressure to afford ethyl 2-(oxetan-3-ylidene)acetate as a colorless viscous oil (815 mg, 79% yield). [00346] 1H NMR (400 MHz, CDCl3): δ 1.29 (t, 3H), 4.18 (q, 2H), 5.32-5.34 (m, 2H), 5.52-5.54 (m, 2H), 5.64-5.66 (m, 1H) ppm.

6704-31-0
321 suppliers
$6.00/1g
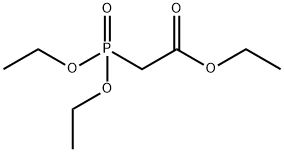
867-13-0
483 suppliers
$10.00/1g

922500-91-2
131 suppliers
$12.00/100mg
Yield:922500-91-2 86%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in tetrahydrofuran at 0 - 20; for 17 h;Cooling with ice;Reagent/catalyst;
Steps:
1; 2; 3; 4; 5 Preparation of compound III
Triethyl phosphonoacetate (932.6g, 4.16mol, 1.5eq.),Put THF (10L) into the reaction flask,Cool down to 0-5C,Add DBU (633.8g, 4.16mol, 1.5eq.) in batches,Stir and react for 30min under ice bath,A large amount of solid precipitated.Under the ice bath,Compound II (200.0g, 2.77mol, 1.0eq.) was added dropwise,The addition is complete.The reaction was stirred at room temperature for 17h.GC detection showed that the reaction of the raw materials was complete. Pour the reaction liquid into 5L saturated sodium carbonate solution, separate the liquids, extract the aqueous phase with EA, combine the organic phases, concentrate under reduced pressure to remove most of the solvent, wash with brine once, dry over magnesium sulfate, and concentrate under reduced pressure. Distillation, collect the fractions at 48-50degC to obtain 338.6g of compound III as colorless oil, yield 86%
References:
Nanjing Furunkaide Bio-pharmaceutical Co., Ltd.;Lian Huawen;Zhang Jie;Liu Guihua CN111848552, 2020, A Location in patent:Paragraph 0051-0054; 0065-0068; 0079-0082; 0092-0095; 0106

6704-31-0
321 suppliers
$6.00/1g

1099-45-2
371 suppliers
$6.00/5g

922500-91-2
131 suppliers
$12.00/100mg

6704-31-0
321 suppliers
$6.00/1g

922500-91-2
131 suppliers
$12.00/100mg