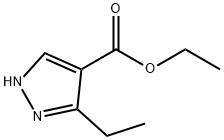
ETHYL-3-ETHYLPYRAZOLE-4-CARBOXYLATE synthesis
- Product Name:ETHYL-3-ETHYLPYRAZOLE-4-CARBOXYLATE
- CAS Number:73981-23-4
- Molecular formula:C8H12N2O2
- Molecular Weight:168.19
![Pentanoic acid, 2-[(dimethylamino)methylene]-3-oxo-, ethyl ester](/CAS/20180527/GIF/89193-23-7.gif)
89193-23-7

73981-23-4
Ethyl 2-((dimethylamino)methylene)-3-oxovalerate (13.9 mmol, 2.76 g) was used as a raw material and reacted with hydrazine (13.9 mmol, 0.44 mL) in ethanol (50 mL) with stirring for 1 h at room temperature. After completion of the reaction, the solvent was removed by evaporation to obtain the crude product. The crude product was purified by rapid chromatography on silica gel using cyclohexane/ethyl acetate (80:20) as eluent. After purification, the eluent was evaporated to afford ethyl 3-ethyl-1H-pyrazole-4-carboxylate (6.00 mmol, 1.01 g, 43% yield) as a yellow oil. The product was analyzed by liquid chromatography (Zorbax SB-C18, 3.5 μm, 4.6 × 50 mm column): retention time (RT) = 1.53 min; mass spectrum (MS) m/z ES+ = 169.
![Pentanoic acid, 2-[(dimethylamino)methylene]-3-oxo-, ethyl ester](/CAS/20180527/GIF/89193-23-7.gif)
89193-23-7
2 suppliers
inquiry

73981-23-4
35 suppliers
inquiry
Yield:73981-23-4 43%
Reaction Conditions:
with hydrazine in ethanol at 20; for 1 h;
Steps:
12
Ethyl 3-ethyl-lH-pyrazole-4-carboxylateAccording to Scheme 9 Step 2: A solution of ()-ethyl 2-((dimethylamino)methylene)- 3-oxopentanoate (13.9 mmol, 2.76 g) and hydrazine (13.9 mmol, 0.44 mL) in EtOH (50
References:
WO2009/10455,2009,A2 Location in patent:Page/Page column 62-63