
ethyl (3R)-4-benzylMorpholine-3-carboxylate synthesis
- Product Name:ethyl (3R)-4-benzylMorpholine-3-carboxylate
- CAS Number:106910-85-4
- Molecular formula:C14H19NO3
- Molecular Weight:249.31

106973-39-1
0 suppliers
inquiry

106910-85-4
11 suppliers
inquiry
Yield:106910-85-4 68%
Reaction Conditions:
Stage #1: ethyl (R)-4-benzyl-5-oxomorpholine-3-carboxylatewith dimethylsulfide borane complex in tetrahydrofuran at 0 - 20;
Stage #2: with water in tetrahydrofuran;
Steps:
46
The title Compound was prepared according to the procedure used by G. R. Brown, J. Chem. Soc. Perkin Trans 1 , 1985, 2577, using ethyl (3f?)-5-oxo-4-(phenylmethyl)- 3-morpholinecarboxylate (D45, 3.64g, 13.84mmol) in dry THF (7OmL), cooled in an ice bath before adding borane-dimethylsulphide complex (1.84mL, 10M), slowly. The reaction mixture was allowed to warm up to room temperature overnight. Stirred for further six hours, added more borane-dimethylsulphide complex and stirred at R.T. over the weekend. Reaction quenched carefully with water, dropwise till the effervescence had finished. Solvent evaporated. Residue dissolved in water, basified to pH ~10 using 2N NaOH solution before extracting with Et2O. Organic layer was then extracted with 2N HCI solution. Acid layer was then basified using 2N NaOH solution before extracting with Et2O x 3. Combined extracts were dried (MgSO4). Filtered. Evaporated under reduced pressure to afford the title compound as a colurless oil in a 68% yield.MS (API+): m/z 250.1 (MH+: 100%) (at) retention time 1.88 min [α]D = + 103°, where I = 1cm, c = 0.5 in DCM
References:
WO2007/28654,2007,A1 Location in patent:Page/Page column 65
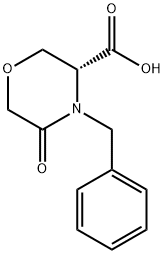
106973-36-8
69 suppliers
$17.00/250mg

106910-85-4
11 suppliers
inquiry

100-52-7
973 suppliers
$20.37/250g

106910-85-4
11 suppliers
inquiry
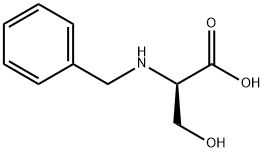
106910-77-4
79 suppliers
$8.00/100mg

106910-85-4
11 suppliers
inquiry