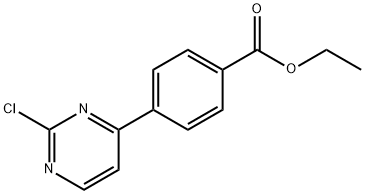
ethyl 4-(2-chloropyriMidin-4-yl)benzoate synthesis
- Product Name:ethyl 4-(2-chloropyriMidin-4-yl)benzoate
- CAS Number:499195-60-7
- Molecular formula:C13H11ClN2O2
- Molecular Weight:262.69
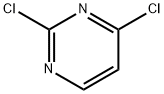
3934-20-1
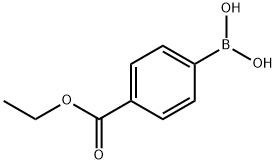
4334-88-7

499195-60-7
Example 1 - Synthesis of Compound 3: A mixture of 4-ethoxycarbonylphenylboronic acid (23.11 g, 119 mmol), 2,4-dichloropyrimidine (16.90 g, 113 mmol), toluene (230 mL), and 2M aqueous sodium carbonate (56 mL) was placed in a reaction flask, vigorously stirred and bubbled under a nitrogen atmosphere for 15 minutes. Tetrakis(triphenylphosphine)palladium [0] (2.61 g, 2.26 mmol) was then added and continued to bubble under nitrogen atmosphere for 10 minutes. The reaction mixture was heated to 100 °C and then lowered to 75 °C for overnight reaction. After completion of the reaction, it was cooled to room temperature, diluted with ethyl acetate (200 mL) and water (100 mL) was added to separate the organic and aqueous layers. The aqueous layer was extracted with ethyl acetate (100 mL) and the organic phases were combined. The organic phase was washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated. The resulting solid was ground with methanol (100 mL), filtered, and washed with methanol (2 x 30 mL) and air dried. The dried solid was dissolved in acetonitrile (150 mL) and dichloromethane (200 mL), and MP.TMT Pd-clearing resin (7.5 g) was added and stirred for 2 days. The reaction mixture was filtered, the solid was washed with dichloromethane (2 x 100 mL), the filtrates were combined and concentrated to give ethyl 4-(2-chloropyrimidin-4-yl)benzoate as a white solid (17.73 g, 60% yield). Further washing with dichloromethane afforded an additional 1.38 g and 0.5 g of product. The products were characterized by 1H NMR (300 MHz, DMSO-d6) and LC-ESI-MS (Method B), and the results were consistent with the expected structure.

3934-20-1
725 suppliers
$9.00/1g

4334-88-7
286 suppliers
$6.00/1g

499195-60-7
51 suppliers
$37.00/100mg
Yield:499195-60-7 60%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in toluene at 75 - 100;Suzuki Coupling;
Steps:
1
Example 1 - Synthesis of Compound 3; A mixture of 4-ethoxycarbonylphenyl boronic acid (23.11 g, 119 mmol), 2,4- dichloropyrimidine (16.90 g, 113 mmol), toluene (230 mL) and aqueous sodium carbonate (2 M, 56 mL) was stirred vigorously and nitrogen was bubbled through the suspension for 15 minutes. Tetrakis(triphenylphosphine)palladium[0] (2.61 g, 2.26 mmol) was added. Nitrogen was bubbled through for another 10 min., the mixture was heated to 100°C, then at 75°C overnight. The mixture was cooled, diluted with ethyl acetate (200 mL), water (100 mL) was added and the layers were separated. The aqueous layer was extracted with ethyl acetate (100 ml) and the two organic extracts were combined. The organics were washed with brine, filtered through sodium sulfate,
References:
WO2008/109943,2008,A1 Location in patent:Page/Page column 56-57