
Ethyl 4-MethanesulfonylcinnaMate synthesis
- Product Name:Ethyl 4-MethanesulfonylcinnaMate
- CAS Number:137473-27-9
- Molecular formula:C12H14O4S
- Molecular Weight:254.3
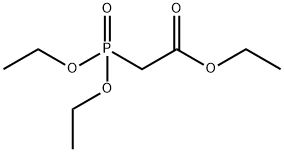
867-13-0
483 suppliers
$10.00/1g
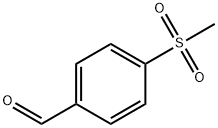
5398-77-6
336 suppliers
$11.00/25g

137473-27-9
20 suppliers
$150.00/250mg
Yield:137473-27-9 87%
Reaction Conditions:
Stage #1: diethoxyphosphoryl-acetic acid ethyl esterwith sodium hydride in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: para-methanesulfonylbenzaldehyde in tetrahydrofuran;N,N-dimethyl-formamide at 0 - 20; for 2 h;
Steps:
4 Ethyl (E)-3-(4-methylsulfonyl)phenyl-2-propenoate
In a solution of sodium hydride (60% in mineral oil, 9.20 g, 0.230 mol) in tetrahydrofuran (250 ml),A solution of ethyl diethylphosphonoacetate (46.2 ml 0.233 mol) in tetrahydrofuran (180 ml) was added dropwise at 0°C and stirred at the same temperature for 10 minutes.To this solution, add the 4-methylsulfonylbenzaldehyde (40.4 g, 0.219 mol) dimethylformamide (300 ml) to the tetrahydrofuran (50 ml) solution obtained in Production Example 3 at 0°C. The mixture was stirred at room temperature for 2 hours. The mixture is ice-cooled again, an aqueous solution of ammonium chloride (25 g) (1.0 1) is added, and thenThe organic layer extracted with ethyl acetate was washed with saturated brine, dried over magnesium sulfate, and concentrated under reduced pressure. By recrystallizing the remnant solid from hexane-isopropyl ether, the title compound was obtained as colorless crystals (48.8 g, yield 87%).
References:
WO1991/12237,1991,A1 Location in patent:Page/Page column 47-48
97410-25-8
3 suppliers
inquiry
4114-31-2
248 suppliers
$5.00/10g

137473-27-9
20 suppliers
$150.00/250mg

5398-77-6
336 suppliers
$11.00/25g

137473-27-9
20 suppliers
$150.00/250mg
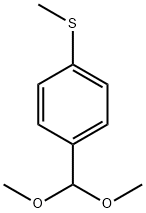
100515-17-1
4 suppliers
inquiry

137473-27-9
20 suppliers
$150.00/250mg
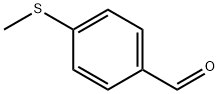
3446-89-7
235 suppliers
$14.00/1g

137473-27-9
20 suppliers
$150.00/250mg