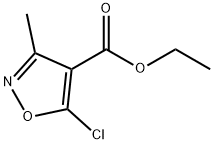
Ethyl 5-chloro-3-methyl-isoxazole-4-carboxylate synthesis
- Product Name:Ethyl 5-chloro-3-methyl-isoxazole-4-carboxylate
- CAS Number:3356-94-3
- Molecular formula:C7H8ClNO3
- Molecular Weight:189.6
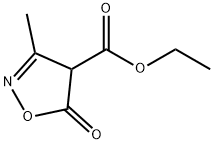
16880-46-9

3356-94-3
General procedure for the synthesis of ethyl 5-chloro-3-methyl-3-isoazole-4-carboxylate from compound (CAS:16880-46-9): to compound 139a (3 g, 17.5 mmol) was added trichlorophosphorus (POCl3, 21.5 g, 140.2 mmol, 13 ml) in a single step. Theophylline (1.8 g, 17.5 mmol) was subsequently added. The reaction mixture was stirred at 110 °C for 24 h under the protection of nitrogen (N2). After completion of the reaction, ice water (15 ml) was added to the mixture to quench the reaction and the aqueous phase was extracted with ethyl acetate (EtOAc, 25 ml x 3). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate (Na2SO4), and concentrated under reduced pressure to give the intermediate compound 139b (brown oil, 2.6 g, 13.7 mmol, yield: 78.2%).1H NMR (400 MHz, CDCl3) δ 4.36 (q, J = 7.1 Hz, 2H), 2.48 (s, 3H). 1.38 (t, J = 7.2Hz, 3H).

16880-46-9
0 suppliers
inquiry

3356-94-3
44 suppliers
$95.00/50mg
Yield:3356-94-3 78.2%
Reaction Conditions:
with triethylamine;trichlorophosphate at 110; for 24 h;Inert atmosphere;
Steps:
81
[0862] to compound 139a (3 g, 17.5 mmol) was added pocl3 (21.5 g, 140.2 mmol, 13 ml) in one portion. Then tea (1.8 g, 17.5 mmol) were added. The mixture was stirred at 110 °C for 24 hours under N2. Then ice water (15 ml) was added in to the mixture, and the aqueous phase was extracted with EtOAc (25 ml x 3), the combined organic layer was washed with brine, dried over Na2SO4, and concentrated to give intermediate compound 139b (2.6 g, 13.7 mmol, yield: 78.2%) as brown oil. 1H NMR (400mhz, CDCl3) δ 4.36 (q, = 7.1 hz, 2h), 2.48 (s, 3h), 1.38 (t, 7 = 7.2 hz, 5h).
References:
WO2018/64119,2018,A1 Location in patent:Paragraph 0862
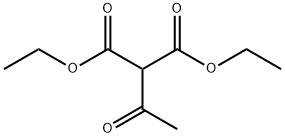
570-08-1
120 suppliers
$30.00/5g

3356-94-3
44 suppliers
$95.00/50mg