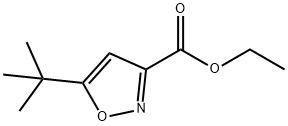
ETHYL 5-TERT-BUTYLISOXAZOLE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 5-TERT-BUTYLISOXAZOLE-3-CARBOXYLATE
- CAS Number:91252-54-9
- Molecular formula:C10H15NO3
- Molecular Weight:197.23

94122-76-6

91252-54-9
General procedure for the synthesis of ethyl 5-tert-butyl-isoxazole-3-carboxylate from the compound (CAS:94122-76-6): tert-butyl alkenone (50) (6.00 g, 30.0 mmol) was dissolved in anhydrous ethanol/THF (1:1, 70 mL) and stirred at room temperature. To this solution was added hydroxylamine hydrochloride (2.29 g, 33.0 mmol) and the resulting mixture was stirred under nitrogen protection for 12 hours. Subsequently, the mixture was transferred to a Soxhlet extractor fitted with molecular sieves and heated to reflux for 2 hours. After completion of the reaction, the mixture was allowed to cool and the solvent was removed by evaporation. Water (100 mL) was added and the mixture was extracted with dichloromethane (2 x 100 mL). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica gel column chromatography to afford ethyl 5-tert-butyl-isoxazole-3-carboxylate (54) as a colorless oil (3 g, 51% yield).1H NMR (500 MHz, CDCl3) data for 54: δ 6.37 (s, 1H), 4.43 (q, 2H), 1.41 (t, 3H), 1.37 (s, 9H).
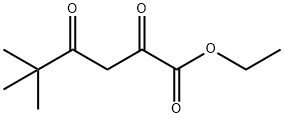
13395-36-3
101 suppliers
$13.43/250mgs:

91252-54-9
82 suppliers
$55.00/250 mg
Yield: 65%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydrogencarbonate in ethanol for 16 h;Reflux;
Steps:
B.1
The title compound is prepared by those skilled in the art according to a literature procedure (Lepage et al, Eur. J. Med. Chem., 1992, 27, 6, 581-93).To a solution of sodium hydrogen carbonate (2.10 g, 25 mmol) and hydroxylamine hydrochloride (1.73 g, 25 mmol) in ethanol (25 mL) is added ethyl trimethyl acetopyruvate (5.00 g, 25 mmol). The mixture is heated at reflux for 16 h. After this time the sodium chloride is removed by filtration and the filtrate is concentrated under reduced pressure. The residue is purified by chromatography on silica eluting with 8/2 cyclohexane/ethyl acetate to provide the title compound as a yellow oil (3.2 g, 65%), m/z 198 [M+H+]. 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 1.38 (9 H, s), 1.42 (3 H, t, /=7.15 Hz), 4.44 (2 H, q, /=7.15 Hz), 6.38 (1 H, s).
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG WO2009/140089, 2009, A2 Location in patent:Page/Page column 37

94122-76-6
1 suppliers
inquiry

91252-54-9
82 suppliers
$55.00/250 mg
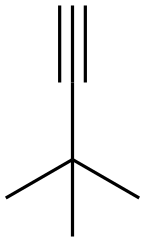
917-92-0
344 suppliers
$23.00/1g
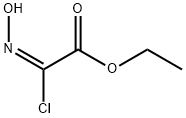
861135-87-7
4 suppliers
$681.00/500g

91252-54-9
82 suppliers
$55.00/250 mg
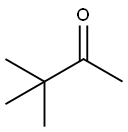
75-97-8
245 suppliers
$10.00/1g
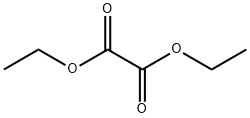
95-92-1
439 suppliers
$5.00/5 g

91252-54-9
82 suppliers
$55.00/250 mg

75-97-8
245 suppliers
$10.00/1g

91252-54-9
82 suppliers
$55.00/250 mg