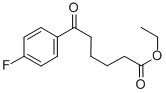
ETHYL-6-(4-FLUOROPHENYL)-6-OXOHEXANOATE synthesis
- Product Name:ETHYL-6-(4-FLUOROPHENYL)-6-OXOHEXANOATE
- CAS Number:327189-51-5
- Molecular formula:C14H17FO3
- Molecular Weight:252.28
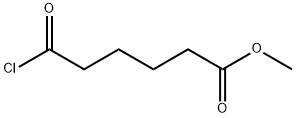
35444-44-1
79 suppliers
$16.00/1g

327189-51-5
8 suppliers
$303.00/1g
Yield:327189-51-5 57%
Reaction Conditions:
with sodium bicarbonate;aluminium trichloride in fluorobenzene;
Steps:
R.6 Reference Example 6
Reference Example 6 To a mixture of fluorobenzene (37.3 g) and adipic acid monoethyl ester chloride (18.0 g) was added gradually aluminum chloride (26.6 g) with stirring under ice-cooling, and the resulting mixture was stirred at room temperature for 2 hours. The reaction mixture was poured into ice water (500 ml) and extracted with diethyl ether (200 ml*2). The organic layer was washed with a 10% aqueous solution of sodium hydrogen carbonate (200 ml), dried over anhydrous magnesium sulfate, and concentrated to give ethyl 6-(4-fluorophenyl)-6-oxohexanoate as an oil (14.1 g, 57%). NMR(CDCl3) δ: 1.25(3H, t, J=7 Hz), 1.6-1.9 (4H, m), 2.30-2.40(2H, m), 2.90-3.00.(2H, m), 4.13(2H, q, J=7 Hz), 7.05-7.15(1H, m), 8.00-8.10 (1H, m).
References:
US6605629,2003,B1
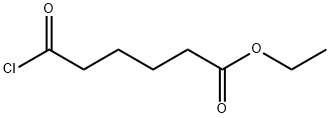
1071-71-2
30 suppliers
$245.00/100mg
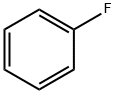
462-06-6
432 suppliers
$10.00/5g

327189-51-5
8 suppliers
$303.00/1g