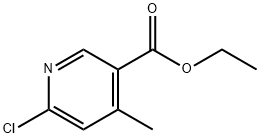
Ethyl 6-chloro-4-methylnicotinate synthesis
- Product Name:Ethyl 6-chloro-4-methylnicotinate
- CAS Number:57591-95-4
- Molecular formula:C9H10ClNO2
- Molecular Weight:199.63
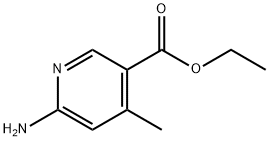
1094345-72-8
0 suppliers
inquiry

57591-95-4
5 suppliers
inquiry
Yield:57591-95-4 31%
Reaction Conditions:
Stage #1: ethyl 6-amino-4-methyl-3-pyridinecarboxylatewith hydrogenchloride;sodium nitrite in water at -7 - 20; for 16.5 h;
Stage #2: with sodium hydroxide in water; pH=12;
Steps:
31.B
To a solution of ethyl β-amino^-methyl-S-pyridinecarboxylate (0.37 g, 2.05 mmol, 1 eq) in 37% HCI (5 ml.) was added a solution of sodium nitrite (0.37 g, 5.3 mmol) in water (3 ml.) dropwise with stirring and cooling such that the temperature did not rise above -5 °C. This was followed by portionwise addition of copper(l) chloride, again maintaining temperature below -5 °C. The resultant mixture was stirred 30 min at -7 °C, warmed to ambient temperature and stirred 16 h. A duplicate reaction was run on a second batch of ethyl 6-amino-4-methyl-3-pyridinecarboxylate (0.80 g, 4.4 mmol) under the same conditions and stoichiometry. These two reactions were combined and poured onto a mixture of ice and diethyl ether. The pH was adjusted to >12 with 6 N NaOH and the phases separated. The aqueous phase was extracted twice with diethyl ether, the organic phases combined, dried over magnesium sulfate, filtered and concentrated to dryness. The crude product was purified by column chromatography (EtOAc/hexanes) to give the title compound (0.40 g, 2.00 mmol, 31 %). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.75 (s, 1 H), 7.60 (s, 1 H), 4.33 (q, 2 H), 2.55 (s, 3 H), 1.33 (t, 3 H). LC-MS (ES+) m/z 199.98, 201.90 [M+H]
References:
WO2008/157273,2008,A1 Location in patent:Page/Page column 60
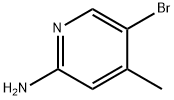
98198-48-2
363 suppliers
$5.00/1g

57591-95-4
5 suppliers
inquiry