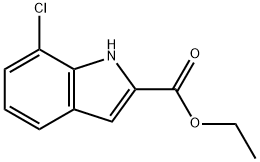
Ethyl 7-chloroindole-2-carboxylate synthesis
- Product Name:Ethyl 7-chloroindole-2-carboxylate
- CAS Number:43142-64-9
- Molecular formula:C11H10ClNO2
- Molecular Weight:223.66
![ethyl 2-[2-(2-chlorophenyl)hydrazinylidene]propanoate](/CAS/20210111/GIF/115050-49-2.gif)
115050-49-2

43142-64-9
General procedure for the synthesis of ethyl 7-chloro-1H-indole-2-carboxylate from the compound (CAS:115050-49-2): 500 g of polyphosphoric acid and 250 g of phosphoric acid were mixed, stirred and heated up to 90°C. Under stirring conditions, 65 g of phenylhydrazine 2-chlorophenylpyruvate pyruvic acid was slowly added and the temperature of the reaction was controlled to be between 90°C and 100°C. The reaction took about 1 hour. The dosing process takes about 1 hour. After completion of dosing, the reaction temperature was maintained to continue the reaction for 20 minutes and the reaction progress was monitored by TLC. Upon completion of the reaction, 1500 g of ice-water mixture was slowly added to quench the reaction and cooled to room temperature. The precipitate was collected by filtration, washed with water and dried to give a light yellow solid product. Upon drying, 51.3 g of ethyl 7-chloro-1H-indole-2-carboxylate was obtained in 85% yield.
![ethyl 2-[2-(2-chlorophenyl)hydrazinylidene]propanoate](/CAS/20210111/GIF/115050-49-2.gif)
115050-49-2
0 suppliers
inquiry

43142-64-9
56 suppliers
$49.00/250mg
Yield: 85%
Reaction Conditions:
with phosphoric acid at 90 - 100; for 1.33 h;Green chemistry;Fischer Indole Synthesis;
Steps:
6.2
500 grams of polyphosphoric acid and 250 grams of phosphoric acid mixed, stirring heated to 90 ° C under the conditions of the batch of itsAdd the raw material 2-chlorophenylhydrazine pyruvate phenyl hydrazone 65 grams, control the temperature range of 90 ~ 100 , about 1 hour to complete the raw materials, continue to control the temperature reaction for 20 minutes, the monitoring reaction is complete, into 1500 grams of ice water mixture , Cooled, filtered and dried to give a pale yellow solid product which was dried to give 51.3 g of ethyl 7-chloroindole-2-carboxylate in 85% yield
References:
China Agricultural University;Huang Jiaxing;Xie Xiaoping CN104402795, 2017, B Location in patent:Paragraph 0060; 0062
617-35-6
558 suppliers
$5.00/10g
41052-75-9
257 suppliers
$5.00/5g

43142-64-9
56 suppliers
$49.00/250mg

617-35-6
558 suppliers
$5.00/10g
95-51-2
389 suppliers
$5.00/5 g

43142-64-9
56 suppliers
$49.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

43142-64-9
56 suppliers
$49.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

43142-64-9
56 suppliers
$49.00/250mg