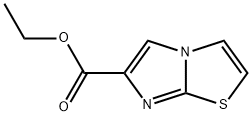
ETHYL IMIDAZO[2,1-B][1,3]THIAZOLE-6-CARBOXYLATE synthesis
- Product Name:ETHYL IMIDAZO[2,1-B][1,3]THIAZOLE-6-CARBOXYLATE
- CAS Number:64951-04-8
- Molecular formula:C8H8N2O2S
- Molecular Weight:196.23
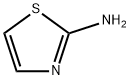
96-50-4
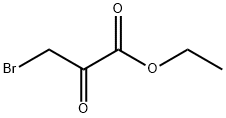
70-23-5
![ETHYL IMIDAZO[2,1-B][1,3]THIAZOLE-6-CARBOXYLATE](/CAS/GIF/64951-04-8.gif)
64951-04-8
General procedure for the synthesis of ethylimidazo[2,1-b]thiazole-6-carboxylic acid from 2-aminothiazole (0.500 g, 5 mmol) and ethyl 3-bromopyruvate (90%, 1.625 g, 7.5 mmol): firstly, 2-amino thiazole was dissolved in tetrahydrofuran, followed by the addition of ethyl 3-bromopyruvate dropwise. The reaction mixture was stirred at room temperature for 8 hours before the precipitate was collected by filtration. The resulting filter cake was dissolved in hot ethanol and refluxed at 80°C for 8 hours. After completion of the reaction, the mixture was cooled and the solvent was removed by distillation. The residual solid was washed with cold water and filtered. After drying under vacuum, the intermediate (0.375 g) was obtained, which was dissolved in tetrahydrofuran. The yield of this step was 38.27%.
Yield:64951-04-8 54%
Reaction Conditions:
in tetrahydrofuran at 25; for 20 h;
References:
Kaizerman, Jacob A.;Gross, Matthew I.;Ge, Yigong;White, Sarah;Hu, Wenhao;Duan, Jian-Xin;Baird, Eldon E.;Johnson, Kirk W.;Tanaka, Richard D.;Moser, Heinz E.;Bürli, Roland W. [Journal of Medicinal Chemistry,2003,vol. 46,# 18,p. 3914 - 3929]